"V体育ios版" Association between polyunsaturated fatty acid intake and allergic rhinitis in children and adolescents: a cross-sectional study of the 2007–2016 NHANES data
Highlight box
Key findings
• High omega-6 (n-6) polyunsaturated fatty acid (PUFA) intake increases the risk of allergic rhinitis (AR), and AR patients have a distinct gut microbiome, with a higher Firmicutes/Bacteroidetes ratio and more pro-inflammatory bacteria.
What is known and what is new?
• AR is a prevalent immune-mediated disease, and its incidence is increasing among children and adolescents VSports在线直播. Previous studies have linked dietary factors, especially PUFAs, to allergic diseases.
• Omega-3 fatty acids have anti-inflammatory properties, while n-6 fatty acids may contribute to inflammation.
What is the implication, and what should change now?
• Reducing the intake of n-6 PUFAs and restoring a balanced gut microbiota may help decrease the incidence of AR, particularly in vulnerable groups like children and adolescents.
Introduction
Allergic rhinitis (AR) is a common immune-mediated disease, defined as a chronic, intermittent, immunoglobulin E-mediated inflammation of the nasal mucosa usually triggered by harmless environmental factors (1). The typical symptoms of AR include nasal congestion, rhinorrhea (anterior and/or posterior), sneezing, and itching. AR significantly affects the quality of life of affected individuals, and in recent years, its global incidence has been steadily increasing, particularly among adolescents, in whom the prevalence of AR is on the rise (1,2). A study has found that among children aged 6 to 12 in Budapest, the prevalence of AR is 14. 9% (3). Sleep-disordered breathing (SDB) is one of the common diseases among children. The most common cause of SDB in children is the hypertrophy of adenoids and tonsils (4). The adenoids are small tissue masses located at the back of the upper airway in the nasal cavity and are part of the immune system. They are largest in children aged 2 to 6. Studies have found that adenoid hypertrophy is closely related to AR V体育官网. On the one hand, adenoid hypertrophy can block the nasal cavity, affecting the ventilation and drainage of the nasal cavity and paranasal sinuses, thereby intensifying the inflammatory response of AR. On the other hand, the inflammatory secretions of AR can repeatedly stimulate the adenoids, leading to further hypertrophy (5). The pathogenesis of AR is complex, involving emotions, genetic, environmental, and dietary factors. Changes in dietary patterns, particularly the increased intake of high-fat and low-fiber diets, are believed to play a significant role in the onset and progression of allergic diseases (6-8).
As research progresses, various nutrients in the diet have been closely linked to the occurrence of AR. Dietary components, especially fatty acids, may play an important role in the development of allergic diseases, particularly in populations with underdeveloped immune systems, such as children (9). Fatty acids, as key components of cell membranes, serve as energy sources and directly affect immune cell function and the regulation of immune responses. The role of polyunsaturated fatty acids (PUFAs) in immune regulation has attracted widespread attention (10-12). A study based on data from the National Health and Nutrition Examination Survey (NHANES) revealed that adults with allergic symptoms had a significantly higher daily intake of PUFAs than healthy individuals, and reported a positive correlation between PUFA intake and allergy symptoms, hay fever, and AR (13). Further, the two main components of PUFAs, omega-3 (n-3) and omega-6 (n-6), may have distinct effects on immune responses. For example, n-3, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), is thought to have anti-inflammatory properties, while n-6 [e. g. , linoleic acid and arachidonic acid (AA)] may play a pro-inflammatory role in allergic reactions (14). An excessively high n-6/n-3 ratio can promote inflammatory responses, increase the risk of AR, and the imbalance of fatty acid ratios may affect the function of immune cells, leading to an overreaction of the immune system to allergens (15). Adolescence is a crucial period for growth and development, with a high demand for nutrients. However, teenagers often prefer fast food and snacks, which are typically rich in n-6 fatty acids but low in n-3 fatty acids (16). Sea fish is an important source of n-3 fatty acids, but many teenagers dislike or seldom consume seafood (17). Moreover, vegetable oils (such as sunflower oil and corn oil) are rich in n-6 fatty acids. Consuming them in large quantities over a long period of time can lead to an imbalance in the n-6/n-3 ratio (18). Gibson et al VSports手机版. conducted a study involving over 23,000 children aged 6 to 15 years. The results showed that as the intake of n-3 and n-6 PUFAs increased, the incidence of eczema also rose (19). From this, it can be seen that the eating habits of teenagers are closely related to the occurrence of AR.
In addition to affecting immune responses, PUFAs, particularly n-3, can indirectly influence the immune system by modulating the composition of the gut microbiota. The gut microbiota plays a crucial role in the function of the immune system, and its dysbiosis is often closely linked to the development of allergic diseases (20). Moreover, nutrients have a profound impact on intestinal microbiota and intestinal immunity. Nutrients and immunity are mediated by intestinal microbiota, and there is a close correlation among these factors (21). More and more evidence indicates that there is a correlation between n-3 PUFAs and the intestinal microbiota. The intake of n-3 PUFAs by the human body affects the diversity of the intestinal microbiota; conversely, the intestinal microbiota also influences the metabolism and absorption of n-3 PUFAs. Studies have found that in adults, changes in the gut microbiota were observed after supplementing with n-3 PUFAs (20). Coincidentally, changes in the intestinal flora have also been observed in patients with intestinal inflammation V体育安卓版. Studies have shown that n-3 can enhance gut barrier integrity and suppress inflammatory responses by altering the structure and metabolic products of the gut microbiota, potentially reducing the occurrence of allergic reactions (14,22). Further, an adequate intake of n-3 may improve gut barrier function by increasing the production of short-chain fatty acids (SCFAs), thereby reducing the incidence of allergic responses (20). Conversely, excessive n-6 intake may exacerbate inflammation by promoting the expansion of specific pathogenic microbial populations, such as Escherichia coli in the gut (22,23). Previous studies have revealed a relationship between dietary PUFAs and AR, and Chen et al. ’s research found that the gut microbiota can regulate the pathological changes and cognitive impairments of Alzheimer’s disease through PUFA (24). However, the interactions between PUFAs and the gut microbiota, especially in the context of AR, remain unclear. Previous research has primarily focused on the relationship between PUFA intake and immune function in adult populations, but research on the association between PUFA intake and gut microbiota abundance in children is limited.
The pathogenesis of AR involves complex immune responses, and its treatment is a systematic process V体育ios版. Many studies have incorporated anti-allergic substances into conventional treatment methods to improve therapeutic efficacy (such as Binahong leaf extract, spleen aminopeptide oral solution) (5,25). Therefore, in order to gain a more comprehensive understanding of the risk factors for childhood AR, it is necessary to further explore the role of PUFA in asthma (AR). This study utilized NHANES data to analyze the differences in PUFA intake between children with asthma aged 1–16 years and the healthy control group. Additionally, by integrating gut microbiota data, this study examined the differences in gut microbiota abundance related to PUFA intake in these two groups. By analyzing the relationship between PUFAs and the gut microbiota, this research aimed to identify potential therapeutic targets, providing new theoretical support for the prevention and treatment of AR. We present this article in accordance with the STROBE reporting checklist (available at https://tp. amegroups. com/article/view/10. 21037/tp-2025-433/rc).
Methods
Study design and population
The NHANES study is a health survey conducted by the Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of the American population. It is carried out every 2 years. This study employs a multi-stage probability sampling method and its data come from cross-sectional surveys based on the population, covering nutrition, overall health status, disease history, and health behaviors. It is a publicly available dataset without personal identifiable information and has been used by researchers worldwide (26).
This study conducted a cross-sectional analysis of the data from the NHANES from 2007 to 2016, involving 3,808 children and adolescents aged 1 to 16 years old. The following 33 variables related to the research purpose were included in the study scope: total saturated fatty acids (TSFAs), total monounsaturated fatty acids (TMFAs), total PUFAs (TPFAs), protein (PROT), energy, carbohydrates (CARBs), vitamins (such as vitamin C, vitamin D, etc.), trace elements (such as zinc, copper), race, and gender, etc. Patients (Figure 1) with missing data on the following variables were excluded from the study: annual household income (167 patients), family income to poverty ratio (FIPR) (3 patients), maternal smoking during pregnancy (409 samples), maternal age during pregnancy (4 patients), height (75 patients), colds (12 samples), gastrointestinal disorders (5 patients), and infections (11 patients). Given the substantial difference in the data between the “rhinitis” and “normal” categories of the dependent variable, the K-nearest neighbors imputation method was first used to impute the missing numerical data to ensure model stability and accuracy (27). Subsequently, random stratified sampling was performed on the “normal” group data to ensure sample representativeness, resulting in 994 valid participants for analysis.
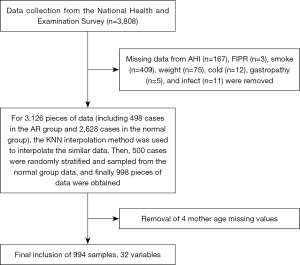
The intestinal gut microbiota table data used in this study originated from the research conducted by Zhang et al. (28). In this study, through the second-generation sequencing technology targeting the V3–V4 region of the 16S ribosomal RNA (rRNA) gene, 16S sequencing and grouping were performed on fecal samples from 24 children with AR and 25 healthy children. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Main variables and outcome variables
The main variables in this study included TPFA (g); total n-3, including octadecatrienoic acid/18:3 (g), octadecatrienoic acid/18:4 (g), EPA/20:5 (g), docosapentaenoic acid/22:5 (g), and DHA/22:6 (g); total n-6, including octadecatrienoic acid/18:2 (g) and eicosatetraenoic acid/20:4 (g); total n-3 intake; total n-6 intake; and the n-3/n-6 ratio. These variables were assessed using data from the Dietary Interview-Individual Foods files and 2-day means from the NHANES database (29).
Similar to previous studies (30-32), in this study, AR status was assessed by self-report. Face-to-face household interviews were conducted, and self-reported allergy, medical conditions, and respiratory health data were collected through an allergy, medical history, and respiratory health questionnaire. Specifically, hay fever was selected as the primary symptom of allergic disease for assessment. The presence of allergic symptoms was confirmed based on participants’ affirmative responses to the following two questions: “Has a doctor ever told you that you have hay fever?” and “In the past 12 months, have you experienced symptoms related to hay fever?”.
"VSports app下载" Potential confounders
Based on previously published literature (29), the potential confounding variables examined in this study included:
- Demographic variables: age (years), sex (male/female), ethnicity (Mexican, other Hispanic, non-Hispanic White, non-Hispanic Black, other race), and the family IPR.
- Clinical variables: hay fever (yes/no), head cold or chest cold (yes/no), gastrointestinal or intestinal disease (yes/no), height (cm), weight (kg), body mass index (BMI; kg/m2), influenza, pneumonia, or ear infections (yes/no).
- Dietary variables: total energy (TE) intake (kcal), PROT (g), total sugar (TSUGR, g), total fat (TTFAT, g), CARB (g), total dietary fiber (TFIBE, g), total saturated fat (TSFAT, g), vitamin A (µg), vitamin (mg), vitamin B12 (µg), vitamin C (mg), vitamin D (µg), vitamin K (µg), zinc (mg), selenium (µg), copper (mg), TSFA (g) (butanoic + hexanoic + octanoic + decanoic + dodecanoic + tetradecanoic + hexadecanoic + octadecanoic), TMFA (g) (hexadecenoic + octadecenoic + eicosenoic + docosenoic), and TPFA (g) (octadecadienoic + octadecatrienoic + octadecatetraenoic + eicosatetraenoic + eicosapentaenoic + docosapentaenoic + docosahexaenoic).
"V体育安卓版" Statistical analysis
To reflect the characteristics of the NHANES multistage sampling survey, a data analysis was performed using R software (version 4.4.1, “survey” and “performance” package) and Python software (version 3.12, “statsmodels” package). Data that followed a normal distribution are expressed as mean and interquartile range (IQR), and the categorical data are expressed as the count and percentage [n (%)]. Group comparisons were conducted using the Mann-Whitney U test (33).
Before fitting the linear mixed-effects model, the intraclass correlation coefficient (ICC) value was calculated using the R package “performance” to assess the random effects of the covariates on AR, and significant random-effects variables were selected based on the ICC value. Subsequently, a generalized linear mixed-effects model (GLMM) was used to further explore the relationship between the covariates and AR. The GLMM combines both fixed and random effects, allowing the estimation of the fixed effect on AR after adjusting for random effects (34). The model construction was as follows: dependent variable: the presence of AR (yes/no); fixed-effect variables: TPFA value; covariates, such as age, sex, BMI, dietary variables (e.g., PUFA intake), ethnicity, family income, etc.; and random effects: PROT, CARB, TSUGR, and TTFAT. The model formula is expressed as follows:
where β represents the fixed-effect parameter, and the term represents the random effect.
The fixed effect estimates the effect of each covariate (e.g., age, sex, and PUFA intake) on the occurrence of AR. We then analyzed the fixed effects of key variables in the GLMM. In addition, univariate logistic regression was used to explore the association between PUFAs, octadecatrienoic acid/18:3, octadecatrienoic acid/18:4, EPA/20:5, docosapentaenoic acid/22:5, DHA/22:6, octadecadienoic acid/18:2, eicosatetraenoic acid/20:4, total n-3 intake, total n-6 intake, or n-3/n-6 ratio, and the risk of AR in children and adolescents. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the relationship between the PUFAs and the risk of AR. All the data are expressed as the mean ± standard deviation. Plotting and statistical analysis were carried out using R software (version 4.4.1). P<0.05 indicates statistical significance.
Results
Comparison of characteristics between adolescents and children with and without AR
The data of 3,808 children and adolescents aged 1 to 16 years from the 2007 to 2016 NHANES were extracted. Missing data included BMI (n=409), family IPR (n=3), maternal smoking during pregnancy (n=409), head cold or chest cold/gastrointestinal diseases/flu, pneumonia, or ear infection (n=28) data. Subsequently, stratified random sampling was performed on the participants without AR to balance selection bias and ensure that the results were representative.
As Table 1 shows, the 994 participants ultimately included in our analysis had an average age of 9 years, an average BMI of 18.17 kg/m2, and 46.4% were male (Table 1). Among the 994 participants, 349 (35.1%) were non-Hispanic White, 201 (20.2%) were Mexican American, 239 (24.0%) were non-Hispanic Black, 100 (10.1%) were other Hispanic, and 105 (10.6%) were of other races. There were significant differences in the AR groups (n=498) in terms of the symptoms (e.g., head cold or chest cold, flu, pneumonia, or ear infections), and the mother’s age during pregnancy. Importantly, significant differences were also observed between the groups in terms of the annual household income and the FIPR.
Table 1
| Variables | Healthy controls (n=496) | AR patients (n=498) | Total study population (n=994) | P value |
|---|---|---|---|---|
| Age (years) | 8.16 [5, 11] | 9.53 [7, 13] | 9 [6, 12] | <0.001*** |
| Male | 254 (51.2) | 207 (41.6) | 461 (46.4) | <0.01** |
| BMI (kg/m2) | 18.91 [15.8, 20.91] | 20.38 [16.23, 22.21] | 18.17 [16, 21.72] | <0.001*** |
| Race | <0.05* | |||
| Non-Hispanic White | 146 (29.4) | 203 (40.8) | 349 (35.1) | |
| Non-Hispanic Black | 131 (26.4) | 108 (21.7) | 239 (24.0) | |
| Mexican American | 121 (24.4) | 80 (16.1) | 201 (20.2) | |
| Other Hispanic | 56 (11.3) | 44 (8.8) | 100 (10.1) | |
| Other race | 42 (8.5) | 63 (12.7) | 105 (10.6) | |
| Head cold or chest cold | 109 (22.0) | 144 (28.9) | 253 (25.5) | <0.05* |
| Gastropathy | 45 (9.1) | 42 (8.4) | 87 (8.8) | 0.74 |
| Flu, pneumonia, ear infection | 17 (3.4) | 39 (7.8) | 56 (5.6) | <0.01** |
| Annual household income | <0.01** | |||
| Under $20,000 | 128 (25.8) | 107 (21.5) | 235 (23.6) | |
| $20,000 to $24,999 | 64 (12.9) | 52 (10.4) | 116 (11.7) | |
| $25,000 to $34,999 | 67 (13.5) | 58 (11.6) | 125 (12.6) | |
| $35,000 to $44,999 | 39 (7.9) | 27 (5.4) | 66 (6.6) | |
| $45,000 to $54,999 | 31 (6.3) | 40 (8.0) | 71 (7.1) | |
| $55,000 to $64,999 | 24 (4.8) | 34 (6.8) | 58 (5.8) | |
| $65,000 to $74,999 | 19 (3.8) | 25 (5.0) | 44 (4.4) | |
| $75,000 to $99,999 | 49 (9.9) | 50 (10.0) | 99 (10.0) | |
| $100,000 and over | 75 (15.1) | 105 (21.1) | 180 (18.1) | |
| FIPR | 1.98 [0.76, 2.95] | 2.33 [0.92, 3.58] | 1.61 [0.81, 3.4] | <0.001*** |
| Mother’s age at infant’s birth (years) | 26.71 [21, 31] | 28.18 [24, 33] | 28 [22, 32] | <0.001*** |
| Maternal smoking during pregnancy | 56 (11.3) | 56 (11.2) | 112 (11.3) | 0.84 |
Data that followed a normal distribution are expressed as mean [IQR], and the categorical data are expressed as n (%). *, P<0.05; **, P<0.01; ***, P<0.001. AR, allergic rhinitis; BMI, body mass index; FIPR, family income to poverty ratio; IQR, interquartile range.
We further examined the differences in dietary components involved in the dietary questionnaire between the two groups (Table 2). In the AR patients, nearly all the dietary components were consumed at higher levels, with significant differences in TE, CARB, TSUGR, vitamin K, and TPFAs. However, there were no significant differences between the two groups in terms of the consumption of vitamin B12, vitamin D, and zinc.
VSports app下载 - Table 2
| Variables | Healthy controls (n=496) | AR patients (n=498) | Total study population (n=994) | P value |
|---|---|---|---|---|
| TE (kcal) | 1,833.08 (1,386.75, 2,128.50) | 1,914.20 (1,434.88, 2,268.63) | 1,773.25 (1,406.25, 2,203.38) | <0.05* |
| PROTs (g) | 69.21 (50.83, 82.55) | 69.61 (51.14, 84.18) | 64.36 (51.06, 83.05) | 0.61 |
| CARBs (g) | 242.60 (183.48, 281.99) | 255.23 (188.27, 298.02) | 238.19 (186.28, 291.26) | <0.05* |
| TSUGRs (g) | 115.54 (81.94, 138.58) | 121.79 (85.53, 150.80) | 110.18 (84.06, 144.98) | <0.05* |
| Dietary fiber (g) | 13.24 (9.09, 16.00) | 13.90 (9.65, 17.35) | 12.40 (9.50, 16.64) | 0.12 |
| TTFAT (g) | 67.26 (46.34, 82.63) | 70.63 (48.46, 86.16) | 63.05 (47.54, 84.20) | 0.06 |
| Vitamin A (mg) | 568.70 (350.13, 714.50) | 594.56 (370.75, 744.13) | 536.00 (360.13, 727.38) | 0.09 |
| Vitamin B6 (mg) | 1.70 (1.15, 2.08) | 1.74 (1.16, 2.16) | 1.56 (1.16, 2.10) | 0.47 |
| Vitamin B12 (mcg) | 4.99 (3.05, 6.18) | 4.88 (3.10, 6.26) | 4.44 (3.06, 6.22) | 0.79 |
| Vitamin C (mg) | 83.26 (38.73, 115.26) | 84.19 (37.63, 115.44) | 68.28 (38.35, 115.56) | 0.62 |
| Vitamin D (D2 + D3) (mcg) | 5.97 (3.25, 8.05) | 5.87 (3.05, 8.00) | 5.43 (3.15, 8.04) | 0.63 |
| Vitamin K (mcg) | 56.37 (26.59, 65.18) | 63.26 (29.64, 75.91) | 43.88 (27.74, 69.46) | <0.05* |
| Zinc (mg) | 10.29 (7.01, 12.66) | 10.08 (7.08, 12.18) | 9.45 (7.06, 12.48) | 0.56 |
| Sodium (mg) | 95.01 (65.76, 114.38) | 95.15 (68.39, 114.15) | 88.58 (67.36, 114.34) | 0.82 |
| Copper (mg) | 0.94 (0.67, 1.11) | 0.97 (0.70, 1.16) | 0.88 (0.68, 1.13) | 0.15 |
| TSFAs (g) | 22.70 (15.11, 27.57) | 23.55 (16.46, 28.47) | 21.17 (15.66, 27.98) | 0.17 |
| TMFAs (g) | 23.21 (15.70, 28.80) | 24.51 (16.66, 29.86) | 21.83 (16.22, 29.31) | 0.07 |
| TPFAs (g) | 13.91 (8.81, 17.91) | 15.17 (9.19, 18.96) | 12.76 (9.00, 18.52) | <0.05* |
| Quartile | <0.01** | |||
| Quartile 1 | 130 (26.2) | 118 (23.7) | 248 (24.9) | |
| Quartile 2 | 143 (28.8) | 105 (21.1) | 248 (24.9) | |
| Quartile 3 | 107 (21.6) | 141 (28.3) | 248 (24.9) | |
| Quartile 4 | 116 (23.4) | 134 (26.9) | 250 (25.2) |
Data that followed a normal distribution are expressed as mean (IQR), and the categorical data are expressed as n (%). *, P<0.05; **, P<0.01. AR, allergic rhinitis; CARB, carbohydrate; IQR, interquartile range; PROT, protein; TE, total energy; TMFA, total monounsaturated fatty acid; TPFA, total polyunsaturated fatty acid; TSFA, total saturated fatty acid; TSUGR, total sugar; TTFAT, total fat.
Construction of a mixed-effects model considering interactions (V体育ios版)
To ensure the significance of the random effects, the ICC values were calculated for TSFAs, TMFAs, TPFAs, PROT, energy, CARB, vitamins (e.g., vitamin C and vitamin D), trace elements (e.g., zinc and copper), and variables like ethnicity and gender, in relation to the presence of AR. The ICC results indicated that the inter-group differences for PROT, CARB, TSUG, and TTFAT were greater than 0.5, while the inter-group differences for the remaining variables were all less than 0.3 in Table 3. Therefore, PROT, CARB, TSUGR, and TTFAT were selected as the random-effects variables for subsequent modeling.
Table 3 (VSports手机版)
| Variables | Sigma | ICC | Residual-Sigma | Residual-ICC |
|---|---|---|---|---|
| BMI | 0.0105 | 0.0419 | 0.2399 | 0.9581 |
| Sex | 0.0042 | 0.0165 | 0.2482 | 0.9835 |
| Age | 0.0113 | 0.0449 | 0.2403 | 0.9551 |
| Race | 0.0064 | 0.0256 | 0.2449 | 0.9744 |
| AHI | 0.0015 | 0.0060 | 0.2488 | 0.9940 |
| FIPR | 0.0267 | 0.1062 | 0.2251 | 0.8938 |
| Mother age | 0.0029 | 0.0115 | 0.2476 | 0.9885 |
| Smoking | 0.0000 | 0.0000 | 0.2503 | 1.0000 |
| Weight | 0.0040 | 0.0158 | 0.2480 | 0.9842 |
| Cold | 0.0031 | 0.0124 | 0.2491 | 0.9876 |
| Gastropathy | 0.0000 | 0.0000 | 0.2503 | 1.0000 |
| Infect | 0.0193 | 0.0722 | 0.2482 | 0.9278 |
| TE | 0.0588 | 0.2350 | 0.1915 | 0.7650 |
| PROT | 0.1649 | 0.6593 | 0.0852 | 0.3407 |
| CARB | 0.2282 | 0.9133 | 0.0217 | 0.0867 |
| TSUGR | 0.1468 | 0.5863 | 0.1036 | 0.4137 |
| TFIBE | 0.0132 | 0.0528 | 0.2371 | 0.9472 |
| TTFAT | 0.1473 | 0.5888 | 0.1029 | 0.4112 |
| TSFAT | 0.1821 | 0.7285 | 0.0679 | 0.2715 |
| Vitamin A | 0.0291 | 0.1162 | 0.2211 | 0.8838 |
| Vitamin B6 | 0.0561 | 0.2241 | 0.1943 | 0.7759 |
| Vitamin B12 | 0.0354 | 0.1416 | 0.2148 | 0.8584 |
| Vitamin C | 0.0719 | 0.2874 | 0.1782 | 0.7126 |
| Vitamin D | 0.0098 | 0.0390 | 0.2406 | 0.9610 |
| Vitamin K | 0.0192 | 0.0769 | 0.2311 | 0.9231 |
| TSFA | 0.1971 | 0.7883 | 0.0530 | 0.2117 |
| TMFA | 0.2181 | 0.8730 | 0.0317 | 0.1270 |
| TPFA | 0.1663 | 0.6645 | 0.0839 | 0.3355 |
| Zinc | 0.0355 | 0.1416 | 0.2149 | 0.8584 |
| Sodium | 0.0328 | 0.1308 | 0.2176 | 0.8692 |
| Copper | 0.0527 | 0.2103 | 0.1977 | 0.7897 |
AHI, annual household income; BMI, body mass index; CARB, carbohydrate; FIPR, family income to poverty ratio; ICC, intraclass correlation coefficient; PROT, protein; TE, total energy; TFIBE, total dietary fiber; TMFA, total monounsaturated fatty acid; TPFA, total polyunsaturated fatty acid; TSFA, total saturated fatty acid; TSFAT, total saturated fat; TSUGR, total sugar; TTFAT, total fat.
Next, possible covariates and interaction terms were selected using a stepwise regression approach. At each step, the P values for each interaction term were calculated, and the final interaction terms were determined based on statistical significance (P<0.05). In our preliminary model, the interaction terms BMI × gastropathy and smoke × TSFA were statistically significant. These interactions were evaluated using fit indices, such as Akaike information criterion (AIC) and Bayesian information criterion (BIC), to confirm that they improved the model fit. As a result, they were included in the final model. The model was constructed with TPFAs as the fixed effect. Each of (1|PROT), (1|CARB), (1|TSUGR), and (1|TTFAT) represents an independent random effect term, with each random effect containing only an intercept, and the following covariates were selected based on significance: TMFA, TPFAs, gender, age, race, cold, infect, and TTFAT. Additionally, BMI × gastropathy and smoke × TSFAs were included as interaction terms, forming a mixed-effects model with random interceptions. The specific model is as follows:
The GLMM was applied, and the significance results of each variable are shown in Table 4. The results indicate that TSFAs, TMFAs, TPFAs, age, and TTFAT have a significant effect on the likelihood of developing AR. Specifically, the coefficient for TTFAT was −29.294 (estimate <0), indicating that an increase in TTFAT reduces the probability of developing AR, while increases in TSFAs, TMFAs, TPFAs, and age increase the probability of developing AR. Compared to females, males have a higher probability of developing AR; compared to Mexican Americans, non-Hispanic Whites and other races have a higher probability of developing AR, with non-Hispanic Whites having the highest likelihood among these groups; compared to individuals without AR, those with a head cold or chest cold (estimate =0.502>0) and those with influenza, pneumonia, or ear infections (estimate =1.039>0) have a higher probability of also having AR. Although individuals with gastrointestinal or intestinal diseases had a lower probability of developing AR than normal individuals (estimate =−1.216<0), a higher BMI in individuals with gastrointestinal or intestinal diseases increased their probability of developing AR. For individuals whose mothers smoked during pregnancy, an increase in TSFA reduced the likelihood of developing AR, but the effect of this variable was less pronounced than that of other variables. The detailed ORs for the effect of each independent variable on the dependent variable (the presence of AR) are shown in Figure 2 and Table 5.
Table 4 (V体育安卓版)
| Terms | Estimate (SD) | 95% CI |
|---|---|---|
| (Intercept) | −2.186 (0.334)*** | −2.840, −1.533 |
| TSFA | 12.453 (4.523)** | 3.589, 21.317 |
| TMFA | 12.322 (4.532)** | 3.440, 21.205 |
| TPFA | 9.576 (3.156)** | 3.391, 15.762 |
| Gender (male) | 0.393 (0.154)* | 0.091, 0.694 |
| Age | 1.186 (0.313)*** | 0.572, 1.799 |
| Race: non-Hispanic Black | 0.251 (0.231) | −0.203, 0.704 |
| Race: non-Hispanic White | 0.930 (0.224)*** | 0.491, 1.369 |
| Race: other Hispanic | 0.263 (0.292) | −0.310, 0.836 |
| Race: other race | 0.856 (0.294)** | 0.279, 1.433 |
| Cold (yes) | 0.502 (0.185)** | 0.138, 0.865 |
| Infect (yes) | 1.039 (0.367)** | 0.321, 1.758 |
| TTFAT | −29.294 (10.400)** | −49.677, −8.910 |
| BMI | 1.056 (0.653) | −0.223, 2.335 |
| Gastropathy (yes) | −1.216 (0.521)* | −2.238, −0.194 |
| Smoked (yes) | 0.783 (0.553) | −0.301, 1.867 |
| BMI × gastropathy (yes) | 6.296 (2.647)* | 1.108, 11.483 |
| TSFA × smoke (yes) | −3.795 (2.003)* | −7.721, 0.130 |
*, P<0.05; **, P<0.01; ***, P<0.001. BMI, body mass index; CI, confidence interval; GLMM, generalized linear mixed-effects model; SD, standard deviation; TMFA, total monounsaturated fatty acid; TPFA, total polyunsaturated fatty acid; TSFA, total saturated fatty acid; TTFAT, total fat.
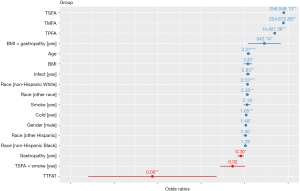
Table 5
| Terms | Estimate (SD) | 95% CI |
|---|---|---|
| (Intercept) | –2.19 (0.33)*** | −2.84, −1.53 |
| TSFA | 12.45 (4.52)** | 3.59, 21.31 |
| TMFA | 12.32 (4.53)** | 3.44, 21.21 |
| TPFA | 9.58 (3.16)** | 3.39, 15.76 |
| Gender (male) | 0.39 (0.15)* | 0.09, 0.69 |
| Age | 1.19 (0.31)*** | 0.57, 1.8 |
| Race: non-Hispanic Black | 0.25 (0.23) | −0.20, 0.70 |
| Race: non-Hispanic White | 0.93 (0.22)*** | 0.49, 1.37 |
| Race: other Hispanic | 0.26 (0.29) | −0.31, 0.84 |
| Race: other race | 0.86 (0.29)** | 0.28, 1.43 |
| Cold (yes) | 0.50 (0.19)** | 0.14, 0.87 |
| Infect (yes) | 1.04 (0.37)** | 0.32, 1.76 |
| TTFAT | −29.29 (10.4)** | −49.68, −8.91 |
| BMI | 1.06 (0.65) | −0.22, 2.36 |
| Gastropathy (yes) | −1.21 (0.52)* | −2.24, −0.19 |
| Smoked (yes) | 0.78 (0.55) | −0.30, 1.87 |
| BMI × gastropathy (yes) | 6.3 (2.65)* | 1.11, 11.48 |
| TSFA × smoke (yes) | −3.8 (2.0) | −7.72, 0.13 |
| SD_(Intercept) PROT | 0.65 | |
| SD_(Intercept) CARB | 0 | |
| SD_(Intercept) TSUGR | 0 | |
| SD_(Intercept) TTFAT | 0.02 |
*, P<0.05; **, P<0.01; ***, P<0.001. BMI, body mass index; CARB, carbohydrate; CI, confidence interval; GLMM, generalized linear mixed-effects model; PROT, protein; SD, standard deviation; TMFA, total monounsaturated fatty acid; TPFA, total polyunsaturated fatty acid; TSFA, total saturated fatty acid; TSUGR, total sugar; TTFAT, total fat.
Fixed-effects analysis (V体育ios版)
We then further analyzed the fixed effects of key variables. The analysis of different types of fatty acids showed that the probability of developing AR was lower at low values of TMFAs and TPFAs, while the probability increased with higher levels of these fatty acids. Moreover, there was a significant difference in the likelihood of developing AR between males and females, with females having a lower probability. Age also had an effect, as older individuals had a higher probability of developing AR. Additionally, there were differences in the probability of developing AR across different ethnic groups, and infections and colds increased the risk of developing AR. The analysis of interaction effects showed that for individuals who self-reported gastrointestinal or intestinal diseases, a higher BMI was associated with an increased risk of developing AR. In the interaction between maternal smoking during pregnancy and TSFAs, the TSFA variable was the main influencing factor, while maternal smoking during pregnancy was nearly unrelated to the risk of developing AR (Figure 3).
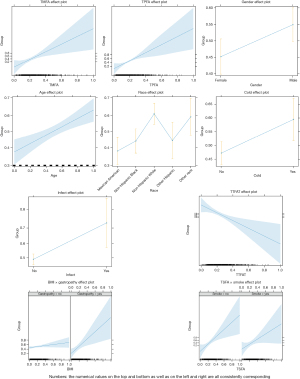
Comparison of TSFA component differences between AR patients and healthy controls in different populations
Previous studies have shown that n-3 PUFAs typically have anti-inflammatory effects, while n-6 PUFAs are associated with pro-inflammatory effects (11,29). Therefore, we further explored the statistical differences in the major components of TPFA between the AR patients and healthy controls. The univariate logistic regression models revealed that in addition to total TPFA intake (OR =1.02; 95% CI: 1.00–1.04; P=0.03), which was associated with an increased risk of developing AR in adolescents and children, the intake of the n-6 component octadecatrienoic acid (OR =1.23; 95% CI: 1.02–1.49; P=0.03) and total n-6 intake (OR =1.02; 95% CI: 1.00–1.04; P=0.03) were also linked to an increased risk of developing AR, further suggesting that total n-6 may promote inflammatory responses in AR patients. Additionally, octadecadienoic acid/18:2 (OR =1.02; 95% CI: 1.01–1.03; P=0.03) was associated with AR, while no significant association was observed between AR and total n-3 (Table 6).
"V体育ios版" Table 6
| Variables | Univariate logistic regression model | |
|---|---|---|
| OR (95% CI) | P value | |
| Octadecadienoic acid/18:2 | 1.02 (1.01–1.03) | 0.03* |
| Octadecatrienoic acid/18:3 | 1.23 (1.02–1.49) | 0.03* |
| Octadecatetraenoic acid/18:4 | 2.58 (0.01–706.61) | 0.74 |
| Eicosatetraenoic acid/20:4 | 0.99 (0.19–5.28) | 0.99 |
| EPA/20:5 | 0.24 (0.01–3.89) | 0.32 |
| Docosapentaenoic acid/22:5 | 0.47 (0.00–139,979) | 0.47 |
| DHA/22:6 | 0.56 (0.12–2.73) | 0.48 |
| TPFA | 1.02 (1.00–1.04) | 0.03* |
| Total n-3 | 1.19 (1.00–1.42) | 0.056 |
| Total n-6 | 1.02 (1.00–1.04) | 0.03* |
| n-3/n-6 | 0.40 (0.01–25.18) | 0.68 |
*, P<0.05. AR, allergic rhinitis; CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; n-3, omega-3; n-6, omega-6; OR, odds ratio; TPFA, total polyunsaturated fatty acid.
Analysis of gut microbiota relative abundance related to PUFAs
Further, increasing evidence indicates a significant correlation between gut microbiota and PUFAs. PUFAs can influence the gut microbiota, while the gut microbiota can affect the metabolism and absorption of PUFAs (7,12,35). Therefore, we hypothesized that a higher intake of total n-6 fatty acids in AR also alters the gut microbiota composition in AR patients. We also incorporated gut microbiota OUT.table data from a study by Zhang et al. (28), which examined AR patients and healthy controls, to explore potential alterations in gut microbiota abundance linked to PUFAs in AR (29). The stacked plot shows that at the phylum level, the Firmicutes/Bacteroidetes (F/B) ratio was significantly higher in the AR group than the healthy control group (Figure 4A). Previous studies suggest that n-3 PUFAs may modulate the proliferation of beneficial bacteria in the gut while inhibiting the growth of harmful bacteria, leading to a decrease in the F/B ratio (7,20).
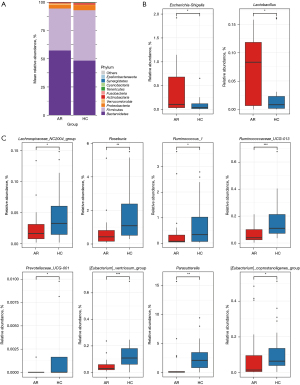
Based on a previously published article (20), we further explored the relationship between gut microbiota abundance and PUFAs in patients with AR. The results revealed an increased relative abundance of Lactobacillus and Escherichia-Shigella in AR, suggesting that gut dysbiosis, potentially induced by excessive intake of n-6 fatty acids, may contribute to heightened immune system activation. This imbalance in the gut microbiota could play a role in exacerbating immune responses in AR patients. Lactobacillus may play a complex role in regulating immune responses, while Escherichia-Shigella may exacerbate allergic reactions due to their pro-inflammatory properties (Figure 4B). Conversely, the healthy controls exhibited a higher abundance of gut microbiota associated with SCFAs, especially butyrate-producing bacteria such as the Lachnospiraceae_NC2004_group, Ruminococcaceae_UCG-013, Roseburia, Prevotellaceae_UCG-001, and Ruminococcus_1 (Figure 4C). These bacteria can promote immune tolerance and exert anti-inflammatory effects, which help alleviate the immune activation response in AR. Therefore, regulating the dietary intake of PUFAs to restore gut microbiota balance, particularly by increasing the abundance of SCFA-producing bacteria, may help reduce the symptoms of AR.
Discussion
Epidemiological studies have shown that the consumption of PUFAs is beneficial to our health, and nutritionists often recommend that patients reduce the ratio of n-6/n-3 fatty acids in their diet (7,10,20,36). Western diets are characterized by a lack of n-3 fatty acids and an excess of n-6 PUFAs. This high n-6/n-3 PUFA ratio shifts enzyme activity toward the use of n-6 PUFAs, which is associated with a heightened pro-inflammatory response (37). This study primarily evaluated the association between the intake of TPFAs and the risk of AR in children and adolescents. The research results indicate that the total intake of PUFA is positively correlated with the risk of developing asthma. Further analysis revealed that compared to n-3 PUFA intake, an increase in n-6 PUFA intake had a greater effect on the risk of AR in children and adolescents. Previous studies have shown that n-3 PUFAs generally exert anti-inflammatory effects, while n-6 PUFAs are associated with pro-inflammatory effects (20). Overall, the findings of this study provide scientific evidence that adolescents and children should reduce their PUFA intake, especially the intake of n-6 PUFAs and octadecadienoic acid/18:2.
In recent years, the role of n-3 and n-6 PUFAs in immune responses has gained increasing attention, particularly their potential effect on AR (20,35,38). N-3 PUFAs, such as EPA and DHA, are believed to alleviate allergic symptoms due to their anti-inflammatory properties. A study has shown that n-3 PUFAs can activate cytosolic calcium accumulation through GPR120, induce the extracellular signal-regulated kinase 1/2 (ERK1/2) mitogen-activated protein kinase (MAPK) signaling pathway, and reduce interleukin (IL)-1β-induced nuclear factor-κB (NF-κB) activation (39). This reduces the production of specific pro-inflammatory cytokines [such as tumor necrosis factor-α (TNF-α), IL-1β, IL-6] to alleviate the immune response and thereby alleviate AR symptoms (7,38). Furthermore, EPA and DHA also produce resolvin and related compounds through pathways involving cyclooxygenase and lipoxygenase. For instance, resolvin E1, resolvin D1, and protectin D1 can inhibit the transendothelial migration of neutrophils, thereby preventing the infiltration of neutrophils at the inflammatory site. Resolvin D1 inhibits the production of IL-1β, while protectin D1 can inhibit the production of TNF and IL-1β (40). Conversely, metabolites of n-6 PUFAs, such as prostaglandin E2 and leukotrienes, can activate the immune system and promote allergic reactions. Studies have shown that treatment with AA and other n-6 PUFAs increased the cytotoxicity (lactate dehydrogenase release) of Calu-3 cells and the secretion of IL-6 (41,42). Studies have shown that stimulation of BEAS-2B cells with AA significantly induced the expression of pro-inflammatory cytokines IL-6 and IL-8. Moreover, the research found that this effect depends on the c-Jun N-terminal kinase (JNK) and p38 MAPK signaling pathways (43). Therefore, the balance of n-3 and n-6 PUFAs in the diet plays a crucial role in regulating immune responses (7,20,44). Moreover, previous Mendelian randomization studies have also shown that higher n-3 PUFA levels are associated with a lower risk of allergic diseases, while n-6 PUFAs are closely associated with an increased risk of certain allergic diseases (22,45). These findings support the results of this study, which suggest that the ratio of n-3 to n-6 PUFAs in the diet may be a key factor in regulating allergic responses. Therefore, adjusting the ratio of n-3 to n-6 PUFA intake in the diet, particularly by reducing the intake of n-6 PUFAs, may help reduce the incidence of AR in children and adolescents.
In recent years, more and more studies have focused on the relationship between PUFAs and gut microbiota, especially their role in allergic diseases (13,46). Existing research has shown that different types of dietary fats, including saturated fatty acids, monounsaturated fatty acids, and PUFAs, as well as their abundance in the diet, can alter the composition of the gut microbiome, thereby affecting immune responses (12,13). The gut microbiome is considered a key regulator of immune responses, especially in maintaining immune tolerance and balance (13,47).
Research on AR has shown that PUFAs, particularly n-3 PUFAs, further modulate the immune system by altering the composition of the gut microbiome and its metabolic products (13,45). For example, n-3 PUFAs can promote the production of SCFAs, such as butyrate, enhance gut barrier function, and reduce intestinal permeability, thereby preventing harmful substances from entering the body (6,48). Moreover, SCFAs promote immune tolerance by regulating T-cell differentiation, thus reducing the occurrence of allergic reactions (7,23,47). This finding is consistent with the observations in this study that the proportion of Bacteroidetes was higher and the proportion of Firmicutes was significantly lower in the AR patients. Similar findings have also been reported in other studies. For instance, a study in the US indicated that adults with nut and pollen allergies had a higher abundance of Bacteroidetes than healthy adults (23,48).
Additionally, a study from Japan found that infants with a higher abundance of Bacteroides were more likely to develop allergic diseases after the age of 2 years (49,50). These studies suggest that Bacteroidetes are closely related to immune system activation and the occurrence of allergic responses, while Firmicutes are typically associated with butyrate production and gut barrier maintenance (6,50,51). Similar findings have been reported in other studies. For instance, a study from the US indicated that the abundance of Bacteroidetes is higher in adults with nut and pollen allergies than healthy adults, and infants with higher Bacteroides abundance are more likely to develop allergic diseases after the age of 2 years (47-49). Additionally, the relative abundance of gut microbiota associated with SCFAs and butyrate is significantly lower in AR patients than healthy controls (52). Among them, we found that Prevotellaceae_UCG-001 was significantly lower in AR patients than in the healthy control group. Bacteria of the genus Prevotella have been found to be common commensal colonizers in mucosal sites and are a core component of one of the three intestinal bacterial enterotypes (53). Prevotella can efficiently ferment dietary fiber to produce beneficial SCFAs, including propionate and acetate. These substances have anti-inflammatory effects, nourish colon cells, and maintain the integrity of the intestinal barrier (54). Moreover, research has found that the abundance of Prevotella decreases in human asthma and chronic obstructive pulmonary disease (COPD), and promotes the growth of Pseudomonas and Lactobacillus. This indicates that the inflammation associated with the disease may directly lead to a reduction in the abundance of Prevotella by creating an unsuitable microenvironment for its survival (55). Therefore, a decrease in the relative abundance of gut microbiota related to SCFAs and butyrate will be detrimental to maintaining a healthy and stable intestinal microbial environment. Further, the relative abundance of Escherichia-Shigella is higher in AR patients than healthy controls; these bacteria directly activate the immune system through their lipopolysaccharide molecules, particularly by triggering strong inflammatory responses via Toll-like receptors. The increased abundance of these bacteria may lead to excessive immune system activation, resulting in the production of pro-inflammatory cytokines, which are closely associated with the typical immune response in AR (56). Overall, excessive n-6 PUFA intake may lead to gut dysbiosis, particularly by promoting the proliferation of pro-inflammatory bacteria while reducing the abundance of beneficial bacteria that produce SCFAs. This imbalance may exacerbate AR symptoms by enhancing the excessive immune response and inflammatory reactions.
This study had some limitations. First, as a cross-sectional study, it can only reveal the correlation between total PUFA intake and AR, and it cannot establish causal relationships. Second, due to the limitations in the NHANES data and the lack of equivalence with the data in the study by Zhang et al. (28), we were unable to explore the relationship between changes in gut microbiome abundance and dietary data further, or to examine the associations between other allergic diseases and changes in gut microbiome abundance in children and adolescents. These limitations may affect the generalizability of the findings. Future prospective cohort studies will help further explore the specific effects of different PUFA components on AR in children and adolescents.
Conclusions (VSports)
Overall, our findings suggest that excessive n-6 PUFA intake is significantly associated with an increased risk of AR in adolescents and children, while the anti-inflammatory effects of n-3 PUFAs may help alleviate the symptoms of AR. The balance of PUFAs (especially n-6 PUFAs) in the diet plays a key role in immune responses and the development of AR. Based on the findings of this study, adjusting the ratio of n-3 to n-6 PUFAs in the diet, particularly by reducing n-6 PUFA intake, may help reduce the incidence of AR. Additionally, the intake of PUFAs is closely related to the abundance of gut microbiota, suggesting that dietary fatty acids may regulate immune system responses by influencing the gut microbiome. Future research should further explore the interactions between PUFAs and the gut microbiome, as well as their specific effect on the pathogenesis of AR, to provide a more scientific basis for the prevention and treatment of AR.
Acknowledgments (V体育官网)
The authors would like to thank Hunan Weishi Biotechnology Research Institute (Changsha, China) for providing theoretical and technical support.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-433/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-433/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-433/coif). H.Z. reports funding support from the Guangdong Provincial Traditional Chinese Medicine Bureau Project (No. 20251248) and the 2024 Guangdong Province Famous Traditional Chinese Medicine Inheritance Studio Construction Project of Lu Ying Famous Traditional Chinese Medicine Inheritance Studio [Yue Zhong Yi Ban Quan (2023) No. 108]. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
"V体育安卓版" References
- Bernstein JA, Bernstein JS, Makol R, et al. Allergic Rhinitis: A Review. JAMA 2024;331:866-77. [Crossref] [PubMed]
- Klimek L, Mullol J, Ellis AK, et al. Current Management of Allergic Rhinitis. J Allergy Clin Immunol Pract 2024;12:1399-412. [Crossref] [PubMed]
- Sultész M, Katona G, Hirschberg A, et al. Prevalence and risk factors for allergic rhinitis in primary schoolchildren in Budapest. Int J Pediatr Otorhinolaryngol 2010;74:503-9. [Crossref] [PubMed]
- Wiltshire D, Ramagiri B, Hira D, et al. Evaluating the relationship between allergic rhinitis and sleep disordered breathing: a retrospective case series. Aust J Otolaryngol 2024;7:33.
- Huang Y, Zhao M, Lin C, et al. Efficacy and safety of spleen aminopeptide oral solution for children with allergic rhinitis and adenoid hypertrophy: a randomised trial. Transl Pediatr 2024;13:1684-95. [Crossref] [PubMed]
- Mann ER, Lam YK, Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol 2024;24:577-95. ["V体育ios版" Crossref] [PubMed]
- Sawane K, Nagatake T, Hosomi K, et al. Dietary Omega-3 Fatty Acid Dampens Allergic Rhinitis via Eosinophilic Production of the Anti-Allergic Lipid Mediator 15-Hydroxyeicosapentaenoic Acid in Mice. Nutrients 2019;11:2868. [Crossref] [PubMed]
- Fakhriani R, Daniswara D, Widuri A. Correlation between anxiety and score for allergic rhinitis. J Xiangya Med 2024;9:3.
- Zhang P. The Role of Diet and Nutrition in Allergic Diseases. Nutrients 2023;15:3683. [Crossref] [PubMed]
- Wang HT, Anvari S, Anagnostou K. The Role of Probiotics in Preventing Allergic Disease. Children (Basel) 2019;6:24. [Crossref] [PubMed]
- Radzikowska U, Rinaldi AO, Çelebi Sözener Z, et al. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019;11:2990. [Crossref] [PubMed]
- Jiao WE, Xi Y, Li D, et al. Association of Dietary Polyunsaturated Fatty Acid Intake with Allergic Rhinitis in Adults: A Cross-Sectional Study of NHANES 2005-2006. Int Arch Allergy Immunol 2024;185:124-32. [Crossref] [PubMed]
- Kaczynska A, Klosinska M, Chmiel P, et al. The Crosstalk between the Gut Microbiota Composition and the Clinical Course of Allergic Rhinitis: The Use of Probiotics, Prebiotics and Bacterial Lysates in the Treatment of Allergic Rhinitis. Nutrients 2022;14:4328. [Crossref] [PubMed]
- Nakamura C, Matsubara A, Nomura A, et al. The relationship of polyunsaturated and monounsaturated fatty acids intake and serum concentrations on inhalant allergen sensitization and allergic rhinitis development. Allergol Int 2025;74:461-7. [Crossref] [PubMed]
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674-88. [VSports注册入口 - Crossref] [PubMed]
- Zhao Y, Wang L, Xue H, et al. Fast food consumption and its associations with obesity and hypertension among children: results from the baseline data of the Childhood Obesity Study in China Mega-cities. BMC Public Health 2017;17:933. [Crossref] [PubMed]
- O'Sullivan TA, Ambrosini G, Beilin LJ, et al. Dietary intake and food sources of fatty acids in Australian adolescents. Nutrition 2011;27:153-9. [Crossref] [PubMed]
- Yang L, Yang C, Chu C, et al. Beneficial effects of monounsaturated fatty acid-rich blended oils with an appropriate polyunsaturated/saturated fatty acid ratio and a low n-6/n-3 fatty acid ratio on the health of rats. J Sci Food Agric 2022;102:7172-85. [Crossref] [PubMed]
- Gibson RS. Principles of Nutritional Assessment. 2nd ed. New York: Oxford University Press; 2005.
- Costantini L, Molinari R, Farinon B, et al. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int J Mol Sci 2017;18:2645. [Crossref] [PubMed]
- Fu Y, Wang Y, Gao H, et al. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediators Inflamm 2021;2021:8879227. [Crossref] [PubMed]
- Lin X, Hu X, Zhang J, et al. Gut microbiota, allergic rhinitis, vasomotor rhinitis, Mendelian randomization, causal association. Braz J Otorhinolaryngol 2024;90:101491. [Crossref] [PubMed]
- Dong L, Tang Y, Wen S, et al. Fecal Microbiota Transplantation Alleviates Allergic Rhinitis via CD4(+) T Cell Modulation Through Gut Microbiota Restoration. Inflammation 2024;47:1278-97. ["VSports app下载" Crossref] [PubMed]
- Chen C, Liao J, Xia Y, et al. Gut microbiota regulate Alzheimer's disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022;71:2233-52. [Crossref] [PubMed]
- Widuri A, Rianto BUD, Indrawati LPL, et al. Effectiveness and safety of addition binahong extract 2.5% on saline nasal irrigation for allergic rhinitis patients: protocol for randomized controlled trial investigator-blinded. J Xiangya Med 2024;9:21.
- Chen TC, Clark J, Riddles MK, et al. National Health and Nutrition Examination Survey, 2015-2018: Sample Design and Estimation Procedures. Vital Health Stat 2 2020;1-35.
- Ren W, Liu Z, Wu Y, et al. Moving Beyond Medical Statistics: A Systematic Review on Missing Data Handling in Electronic Health Records. Health Data Sci 2024;4:0176.
- Zhang P, Zhou X, Tan H, et al. Microbial signature of intestine in children with allergic rhinitis. Front Microbiol 2023;14:1208816. [Crossref] [PubMed]
- Zhang B, Li P, Fu P. Association between Polyunsaturated Fatty Acid Intake and Eczema in Children and Adolescents. Int Arch Allergy Immunol 2023;184:681-91. [Crossref] [PubMed]
- Kong W, Xie Y, Zhong J, et al. Ultra-processed foods and allergic symptoms among children and adults in the United States: A population-based analysis of NHANES 2005-2006. Front Public Health 2022;10:1038141. [Crossref] [PubMed]
- Roxbury CR, Qiu M, Shargorodsky J, et al. Association Between Rhinitis and Depression in United States Adults. J Allergy Clin Immunol Pract 2019;7:2013-20. [V体育安卓版 - Crossref] [PubMed]
- Wu L, Zhang T, Luo W, et al. Rhinitis symptom in patients with self-reported allergic rhinitis is influenced by sensitization pattern: A cross-sectional study of China. Int Forum Allergy Rhinol 2023;13:1007-16. [Crossref] [PubMed]
- Núñez E, Steyerberg EW, Núñez J. Regression Modeling Strategies. Revista Española de Cardiología (English Edition) 2011;64:501-7. [Crossref] [PubMed]
- Mulyaningsih T, Mohanty I, Widyaningsih V, et al. Beyond personal factors: Multilevel determinants of childhood stunting in Indonesia. PLoS One 2021;16:e0260265. [Crossref] [PubMed]
- Lee-Sarwar K, Kelly RS, Lasky-Su J, et al. Dietary and Plasma Polyunsaturated Fatty Acids Are Inversely Associated with Asthma and Atopy in Early Childhood. J Allergy Clin Immunol Pract 2019;7:529-538.e8. [Crossref (VSports app下载)] [PubMed]
- Tabaru A, Ogreden S, Akyel S, et al. Comparison of treatment efficacy of omega-3 fish oil and montelukast in ovalbumin-protease-induced allergic rhinitis model in rats. Braz J Otorhinolaryngol 2024;90:101399. [Crossref] [PubMed]
- Li X, Bi X, Wang S, et al. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front Immunol 2019;10:2241. [Crossref] [PubMed]
- Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 2012;3:1-7. ["V体育官网" Crossref] [PubMed]
- Mobraten K, Haug TM, Kleiveland CR, et al. Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Health Dis 2013;12:101. ["V体育平台登录" Crossref] [PubMed]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349-61. [Crossref] [PubMed]
- Ghadiri M, Mamlouk M, Spicer P, et al. Effect of polyunsaturated fatty acids (PUFAs) on airway epithelial cells' tight junction. Pulm Pharmacol Ther 2016;40:30-8. [Crossref] [PubMed]
- Haghi M, Traini D, Wood LG, et al. A 'soft spot' for drug transport: modulation of cell stiffness using fatty acids and its impact on drug transport in lung model. J Mater Chem B 2015;3:2583-9. [Crossref] [PubMed]
- Rutting S, Zakarya R, Bozier J, et al. Dietary Fatty Acids Amplify Inflammatory Responses to Infection through p38 MAPK Signaling. Am J Respir Cell Mol Biol 2019;60:554-68. [Crossref] [PubMed]
- Sartorio MUA, Pendezza E, Coppola S, et al. Potential Role of Omega-3 Polyunsaturated Fatty Acids in Pediatric Food Allergy. Nutrients 2021;14:152. [Crossref] [PubMed]
- Li Y, Li Q, Cao Z, et al. The causal association of polyunsaturated fatty acids with allergic disease: A two-sample Mendelian randomization study. Front Nutr 2022;9:962787. [Crossref] [PubMed]
- Hu Y, Zhang R, Li J, et al. Association Between Gut and Nasal Microbiota and Allergic Rhinitis: A Systematic Review. J Asthma Allergy 2024;17:633-51. [Crossref] [PubMed]
- Liu X, Tao J, Li J, et al. Dysbiosis of Fecal Microbiota in Allergic Rhinitis Patients. Am J Rhinol Allergy 2020;34:650-60. [Crossref] [PubMed]
- Kim WG, Kang GD, Kim HI, et al. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef Microbes 2019;10:55-67. ["VSports" Crossref] [PubMed]
- Su YJ, Luo SD, Hsu CY, et al. Differences in gut microbiota between allergic rhinitis, atopic dermatitis, and skin urticaria: A pilot study. Medicine (Baltimore) 2021;100:e25091. ["VSports最新版本" Crossref] [PubMed]
- Songjinda P, Nakayama J, Tateyama A, et al. Differences in developing intestinal microbiota between allergic and non-allergic infants: a pilot study in Japan. Biosci Biotechnol Biochem 2007;71:2338-42. [Crossref] [PubMed]
- Chen Y, Guo C, Chung MK, et al. The Associations of Prenatal Exposure to Fine Particulate Matter and Its Chemical Components with Allergic Rhinitis in Children and the Modification Effect of Polyunsaturated Fatty Acids: A Birth Cohort Study. Environ Health Perspect 2024;132:47010. [Crossref] [PubMed]
- Min HK, Na HS, Jhun J, et al. Identification of gut dysbiosis in axial spondyloarthritis patients and improvement of experimental ankylosing spondyloarthritis by microbiome-derived butyrate with immune-modulating function. Front Immunol 2023;14:1096565. [Crossref] [PubMed]
- Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature 2011;473:174-80. [V体育官网 - Crossref] [PubMed]
- Chen T, Long W, Zhang C, et al. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci Rep 2017;7:2594. [Crossref] [PubMed]
- Yadava K, Pattaroni C, Sichelstiel AK, et al. Microbiota promotes chronic pulmonary inflammation by enhancing IL-17a and autoantibodies. Am J Respir Crit Care Med 2016;193:975-87. [Crossref] [PubMed]
- Venter C. Immunonutrition: Diet Diversity, Gut Microbiome and Prevention of Allergic Diseases. Allergy Asthma Immunol Res 2023;15:545-61. [Crossref] [PubMed]


