Clinical characteristics analysis and prediction model construction for pediatric influenza virus pneumonia complicated by bacterial infection
Highlight box
Key findings
• In 1,966 children with influenza virus pneumonia, nine routine variables independently predict bacterial co-infection.
• The logistic model shows high discrimination, good overall accuracy, sound calibration, and decision-curve net benefit; a nomogram enables bedside risk estimation.
What is known and what is new?
• Bacterial co-infection worsens pediatric influenza pneumonia, but early recognition at presentation is challenging and existing tools are limited in scope or practicality.
• We provide a parsimonious, internally validated model built entirely from widely available clinical and laboratory variables, with strong performance and a user-friendly nomogram to support individualized risk assessment.
What is the implication, and what should change now?
• The model can inform early triage, prompt microbiologic testing and timely antibiotics in high-risk patients, and help avoid unnecessary antibiotics in low-risk patients, supporting antimicrobial stewardship. Integration into electronic health records as clinical decision support is feasible; prospective, multicenter impact studies should precede widespread adoption V体育官网.
Introduction
Influenza virus is a major pathogen responsible for the global burden of respiratory infections, particularly among children (1,2). Influenza infection in pediatric populations is characterized by a high incidence rate, frequent complications, and rapid disease progression (3). According to data from the World Health Organization (WHO), approximately one billion cases of influenza occur worldwide each year, with 3 to 5 million classified as severe cases, resulting in 290,000 to 650,000 deaths. Children, especially those under 5 years of age, are considered a high-risk group for severe influenza-related complications and with high mortality rate (4). Influenza virus infection can lead not only to upper respiratory tract inflammation but also to lower respiratory tract involvement, causing viral pneumonia (5). In severe cases, it may be complicated by bacterial co-infection, which further exacerbates the disease severity, increases hospitalization and mechanical ventilation rates, and elevates the risk of and high mortality rate (6) VSports手机版.
During influenza virus infection, the integrity of the host’s respiratory epithelium is compromised, and local immune defenses are impaired, creating a favorable environment for bacterial colonization and invasion (7). Common pathogenic bacteria, such as Streptococcus pneumoniae (8), Staphylococcus aureus (9), and Haemophilus influenzae (10), can cause secondary infections following viral damage, forming a typical virus-bacteria synergistic pathogenic mechanism (11) V体育安卓版. A previous study has shown that during influenza pandemics, some critically ill patients experience bacterial co-infection, with an even higher rate observed in children (12). Bacterial co-infection can lead to more severe lung tissue destruction, respiratory failure, sepsis, and multiple organ dysfunction syndrome, significantly worsening disease severity and prognosis, while greatly increasing the complexity of clinical management and healthcare resource utilization (13).
Therefore, this study aims to comprehensively investigate the clinical characteristics of pediatric influenza virus pneumonia complicated by bacterial infection and to establish an effective predictive model. Such a model would facilitate early disease identification, risk stratification, and precision treatment, holding important clinical significance. We present this article in accordance with the TRIPOD reporting checklist (available at https://tp. amegroups. com/article/view/10. 21037/tp-2025-340/rc) V体育ios版.
Methods (VSports)
Study design
This was a single-center, retrospective cohort study.
Study subjects
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. According to the policies of the Ethics Committee of The Affiliated Hospital to Changchun University of Chinese Medicine, this retrospective analysis of pre-existing, fully de-identified clinical data—with no patient contact, intervention, or change in management—was considered minimal risk and did not require formal ethics approval. The requirement for individual informed consent was waived on the grounds of de-identification and impracticability of obtaining consent V体育平台登录.
Pediatric patients diagnosed with influenza viral pneumonia and treated at the Pediatric Diagnosis and Treatment Center to The Affiliated Hospital to Changchun University of Chinese Medicine between January 1, 2022 and May 10, 2025, were retrospectively collected as study subjects. Patients were categorized into two groups based on whether they had a confirmed bacterial co-infection: the non-co-infection group and the bacterial co-infection group. Cases collected from January 2022 to December 2024 were used to construct the predictive model (training set), while cases collected from January to May, 2025 were used as an external validation set.
Bacterial co-infection confirmation
Bacterial co-infection was confirmed based on four standards: (I) microbiological stringency: co-infections were confirmed only when cultures from sterile sites or high-quality respiratory specimens surpassed predefined colony forming unit (CFU) thresholds and demonstrated bacterial presence with phagocytosis on microscopy, thereby ruling out low-level colonization; (II) triggered sampling based on C-reactive protein (CRP) and platelet count (PLT): rather than using biomarkers alone for diagnosis, procalcitonin (PCT) (>0.5 ng/mL) and CRP elevations guided the decision to collect cultures, leveraging their high specificity in pediatric influenza to optimize sampling without overcalling infection; (III) robustness checks: to address concerns about undetected bacterial cases, we excluded culture-negative patients with PCT >0.5 ng/mL or white blood cell count (WBC) >15×109/L in sensitivity analyses; model discrimination [area under the receiver operating characteristic curve (AUC)] and coefficient estimates remained stable, affirming the reliability of our classification; and (IV) combined clinical-laboratory definition: bacterial co-infection required both clinical signs unexplained by influenza alone and microbiological proof via quantitative culture, with strict exclusion of samples indicating contamination or colonization [e.g., >10 squamous epithelial cells/high power field (HPF) or <25 leukocytes/HPF] (14).
Inclusion criteria
- Aged between 1 month and 12 years.
- Diagnosed with influenza infection, confirmed by epidemiological history, clinical symptoms, and positive influenza antigen or nucleic acid test.
- Diagnosed with pneumonia based on clinical, radiological, and laboratory findings.
Exclusion criteria (V体育安卓版)
- Diagnoses of pneumonia due to pathogens other than influenza virus (e.g., Mycoplasma pneumoniae, respiratory syncytial virus, adenovirus).
- Incomplete or missing critical clinical data.
- Co-infection with fungi, atypical bacteria (e.g., Mycoplasma pneumoniae), or other non-bacterial pathogens.
Data collection
Collected variables included: co-infection status, bacterial type, age, sex, pre-hospital fever duration, temperature, WBC, CRP, PCT, PLT, lactate dehydrogenase (LDH), underlying diseases (congenital heart disease, bronchopulmonary dysplasia, asthma, neurological disorders, immunodeficiency, hematologic diseases), gasping, influenza vaccination status, lymphocyte count (LY), neutrophil count (NEU), monocyte count (MO), pulmonary rales, peak fever, and influenza type. In our retrospective cohort, each patient’s age was calculated at the time of hospital admission—that is, the date on which they were first registered in our electronic medical record with a diagnosis of influenza-virus pneumonia (between January 1, 2022 and May 10, 2025). Birthdate and admission date were both documented by nursing staff at entry, and age (in years, to two decimal places) was derived directly from these timestamps. All data were obtained from the hospital’s electronic medical record system. Laboratory test results were recorded as the first measurements upon admission.
Antibiotic methods (VSports app下载)
Empiric antibiotic therapy was initiated for any child exhibiting signs suggestive of bacterial involvement (e.g., high fever, WBC >12×109/L, PCT >0.5 ng/mL, or gasping), typically within 24 hours of hospital admission. Regimens followed institutional pediatric pneumonia guidelines, starting with a broad spectrum β lactam (ceftriaxone) plus a macrolide (azithromycin) when atypical pathogens were suspected. Antibiotic choice and duration were adjusted based on culture results and clinical response. We extracted timing of first dose and total duration of therapy from the electronic medical record for all patients.
Outcome assessment
The primary outcome predicted by the model was the presence or absence of bacterial co-infection in children with influenza virus pneumonia. Outcome assessment was performed retrospectively by experienced pediatric clinicians who were blinded to the predictor variable modeling and statistical analysis process. To reduce potential assessment bias, clinical and laboratory data were reviewed independently from modeling procedures, and bacterial co-infection status was determined prior to statistical modeling by a separate clinical team not involved in data analysis.
Sample size
As this was a retrospective cohort study, no formal sample size calculation was performed prior to data collection. Instead, all eligible pediatric patients diagnosed with influenza virus pneumonia and admitted to the hospital between January 1, 2022 and May 10, 2025, were consecutively included. The sample size was determined by the availability of complete clinical data during this time period. This approach ensured a sufficiently large and representative dataset for both model development (n=1,607) and external validation (n=359).
Data preprocessing (VSports手机版)
To further evaluate our model’s internal robustness beyond the temporal external validation, we implemented two resampling techniques within the 1,607-patient development cohort. First, we performed 5-fold cross-validation: the data were randomly partitioned into five equal subsets, and in each iteration four folds were used to train the logistic regression model while the remaining fold served as the test set; this process was repeated so that each fold acted once as the held-out data. We then averaged the AUC, Brier score, and calibration slope across all five folds. Second, we applied bootstrap optimism correction with 1,000 bootstrap samples: in each sample the model was refit and its performance on both the bootstrap and original datasets was compared to estimate and adjust for over-optimism in discrimination and calibration metrics.
Clinical data, laboratory indicators, and imaging results were extracted from electronic records. Data cleaning involved the removal of duplicates and correction of logical errors. Missing values were handled as follows: if the proportion of missing data for a variable exceeded 30%, the variable was excluded; otherwise, multiple imputation was used. Continuous variables were standardized as necessary. Categorical variables were encoded appropriately for subsequent analysis.
VSports app下载 - Multivariate analysis for variable selection
Prior to selection, all candidate predictors (clinical, laboratory, and imaging variables) were rigorously preprocessed: duplicates were removed, logical inconsistencies corrected, and any variable with >30% missingness was dropped while the remainder underwent multiple imputation; continuous features were then standardized and categorical features appropriately encoded. Thereafter, we performed univariate analyses to screen for associations with bacterial co-infection, retaining those variables with P<0.05 for entry into a multivariate logistic regression model. In the present model, independent predictors were identified and quantified by odds ratios (ORs) and 95% confidence intervals (CIs).
Logistic regression analysis
Based on the results of multivariate analysis, a binary logistic regression model was developed to evaluate the association between selected variables and the risk of bacterial co-infection. Model discrimination was assessed using the area under the AUC. Calibration was evaluated using a calibration curve and the Brier score, and model goodness-of-fit was assessed with the Hosmer-Lemeshow test.
A nomogram was subsequently constructed based on the logistic regression model to provide individualized risk prediction for bacterial co-infection. To further evaluate the model’s clinical applicability, forest plots were used to visualize the ORs of key predictors, calibration curves were used to compare predicted and observed probabilities, and decision curve analysis (DCA) was employed to assess the clinical utility of the model.
"V体育官网入口" Data bias control
To mitigate selection bias inherent in a single-center, retrospective design, we employed a consecutive sampling strategy in which all pediatric patients meeting predefined inclusion criteria (influenza virus pneumonia admitted between January 1, 2022 and May 10, 2025 with complete clinical data) were enrolled without exception. Study variables and case definitions were specified a priori and applied uniformly by trained investigators who extracted data using a standardized electronic case report form. To address potential bias from missing information, we excluded variables with >30% missingness and applied multiple imputation for the remainder, ensuring that no patient was omitted solely due to sporadic data gaps. Furthermore, we conducted sensitivity analyses—such as reclassifying high-PCT/WBC, culture negative cases and excluding early antibiotic recipients—to confirm that our key findings remained robust under alternative assumptions about unmeasured confounding and diagnostic misclassification.
V体育官网入口 - Statistical analysis
All statistical analyses were performed using R version 4.2.3 and Python version 3.11.4. Continuous variables were first tested for normality using the Shapiro-Wilk test. Data following a normal distribution were expressed as mean ± standard deviation (SD), and comparisons between two groups were performed using the independent samples t-test. Data with a skewed distribution were expressed as median [interquartile range (IQR)], and comparisons between two groups were performed using the Mann-Whitney U test. Categorical variables were expressed as n (%), and comparisons were made using the Chi-squared test or Fisher’s exact test. Statistical significance was defined as a P<0.05.
Results
Study population
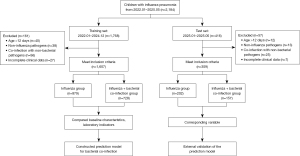
Figure 1 shows the patient selection process. A total of 2,184 children with influenza pneumonia were initially screened between January 2022 and May 2025. After applying the inclusion and exclusion criteria, 218 patients were excluded (161 in the training set and 57 in the test set). The final cohort consisted of 1,926 patients. The training set (n=1,607) included 728 patients in the influenza + bacterial co-infection group and 879 in the influenza group. The test set (n=359) comprised 157 cases in the co-infection group and 202 in the influenza group, and was used for external validation of the prediction model.
Baseline characteristics and clinical indicators
Among the 1,607 children included, 728 were diagnosed with bacterial co-infection and 879 with influenza virus pneumonia alone (Table 1). Compared to the non-co-infection group, the co-infection group had significantly younger age (median: 4 vs. 5 years, P<0.001), higher body temperature (39.20 vs. 38.80 ℃, P<0.001), and markedly elevated inflammatory markers, including WBC (12.70×109/L vs. 11.40×109/L), CRP (23.50 vs. 15.10 mg/L), PCT (0.76 vs. 0.30 ng/mL), PLT (300.45×109/L vs. 249.30×109/L), and LDH (364.45 vs. 342.25 U/L), all with P<0.001. In addition, the co-infection group had higher proportions of children with underlying diseases (32.69% vs. 14.79%, P<0.001) and gasping symptoms (36.54% vs. 25.03%, P<0.001).
VSports - Table 1
| Parameters | All (n=1,607) | Non-co-infection group (n=879) | Bacterial co-infection group (n=728) | P |
|---|---|---|---|---|
| Age (years) | 4.00 (3.00; 6.00) | 5.00 (4.00; 7.00) | 4.00 (3.00; 5.00) | <0.001 |
| Pre-hospital fever duration (days) | 1.80 (1.50; 2.10) | 1.80 (1.50; 2.10) | 1.80 (1.50; 2.20) | 0.53 |
| Temperature (℃) | 39.00 (38.60; 39.40) | 38.80 (38.50; 39.15) | 39.20 (38.80; 39.60) | <0.001 |
| WBC (×109/L) | 11.90 (9.80; 14.10) | 11.40 (8.50; 13.25) | 12.70 (11.00; 14.70) | <0.001 |
| CRP (mg/L) | 16.70 (14.50; 22.90) | 15.10 (13.70; 16.30) | 23.50 (19.80; 27.70) | <0.001 |
| PLT (×109/L) | 274.40 (211.05; 330.85) | 249.30 (197.85; 302.50) | 300.45 (239.93; 368.50) | <0.001 |
| PCT (ng/mL) | 0.51 (0.20; 0.810) | 0.30 (0.12; 0.58) | 0.76 (0.43; 1.12) | <0.001 |
| LDH (U/L) | 352.31±96.56 | 342.25±80.26 | 364.45±112.03 | <0.001 |
| LY (%) | 29.80 (21.90; 37.50) | 29.80 (23.10; 36.80) | 29.80 (20.30; 38.40) | 0.40 |
| NEU (%) | 69.60 (62.90; 76.25) | 70.00 (63.05; 76.95) | 69.20 (62.68; 75.65) | 0.15 |
| MO (×109/L) | 0.70 (0.60; 0.80) | 0.70 (0.60; 0.80) | 0.70 (0.60; 0.80) | 0.56 |
| Peak fever (℃) | 39.20 (38.90; 39.50) | 39.20 (38.90; 39.50) | 39.20 (38.90; 39.60) | 0.34 |
| Sex | 0.30 | |||
| Female | 922 (57.37) | 515 (58.59) | 407 (55.91) | |
| Male | 685 (42.63) | 364 (41.41) | 321 (44.09) | |
| Underlying diseases | <0.001 | |||
| No | 1,239 (77.10) | 749 (85.21) | 490 (67.31) | |
| Yes | 368 (22.90) | 130 (14.79) | 238 (32.69) | |
| Gasping | <0.001 | |||
| No | 1,121 (69.76) | 659 (74.97) | 462 (63.46) | |
| Yes | 486 (30.24) | 220 (25.03) | 266 (36.54) | |
| Antibiotics | <0.001 | |||
| No | 67 (4.17) | 67 (7.62) | 0 | |
| Yes | 1,540 (95.83) | 812 (92.38) | 728 (100.0) | |
| Pulmonary rales | 0.055 | |||
| No | 1,011 (62.91) | 534 (60.75) | 477 (65.52) | |
| Yes | 596 (37.09) | 345 (39.25) | 251 (34.48) | |
| Influenza type | 0.18 | |||
| Influenza A | 1,184 (73.68) | 660 (75.09) | 524 (71.98) | |
| Influenza B | 423 (26.32) | 219 (24.92) | 204 (28.02) | |
| Influenza vaccine | 0.11 | |||
| No | 1,292 (80.40) | 694 (78.95) | 598 (82.14) | |
| Yes | 315 (19.60) | 185 (21.05) | 130 (17.86) |
Data are expressed as median (interquartile range), mean ± standard deviation, or n (%). CRP, C-reactive protein; LDH, lactate dehydrogenase; LY, lymphocyte count; MO, monocyte count; NEU, neutrophil count; PCT, procalcitonin; PLT, platelet count; WBC, white blood cell count.
Empiric antibiotic therapy was administered to nearly all children—even those without confirmed bacterial co-infection—with 92.4% (812/879) of the non-co-infection group and 100% (728/728) of the co-infection group receiving antibiotics (Fisher’s exact P<0.001, Table 1). Moreover, the median duration of therapy was substantially shorter for the non-co-infection cohort at 3 days (IQR, 2–5 days) vs. 8 days (IQR, 6–12 days) in the co-infection cohort (Mann-Whitney U test, P<0.001). These highly significant differences reflect real-world practice: clinicians initiate empiric antibiotics broadly at admission, but confirmatory culture results and clinical response drive a longer course in true co-infection cases while allowing earlier de-escalation when bacterial infection is ruled out.
In contrast, no statistically significant differences were observed between the two groups in terms of pre-hospital fever duration (P=0.53), peak fever (P=0.34), influenza vaccination status (P=0.11), LY% (P=0.40), NEU% (P=0.15), MO% (P=0.56), sex distribution (P=0.30), presence of pulmonary rales (P=0.055), or influenza virus type (P=0.18).
Distribution of bacterial co-infection
Among the 728 patients in the bacterial co-infection group, 78.16% (n=569) were infected with Gram-negative bacteria, and 21.84% (n=159) with Gram-positive bacteria (Table 2). The most common Gram-negative pathogens were Haemophilus influenzae (28.85%), Klebsiella pneumoniae (20.19%), and Moraxella catarrhalis (10.30%). Other Gram-negative organisms included Escherichia coli (6.04%), Pseudomonas aeruginosa (3.98%), Enterobacter cloacae (3.16%), Haemophilus parainfluenzae (0.96%), Acinetobacter baumannii (2.06%), Enterobacter aerogenes (1.37%), and Stenotrophomonas maltophilia (1.24%). Gram-positive pathogens included Streptococcus pneumoniae (9.62%), Staphylococcus aureus (5.77%), Streptococcus viridans (3.16%), Staphylococcus epidermidis (2.47%), and Enterococcus faecium (0.82%).
Table 2 (VSports最新版本)
| Pathogenic bacteria | Type | Cases (n) | Proportion (%) |
|---|---|---|---|
| Haemophilus influenzae | G− | 210 | 28.85 |
| Klebsiella pneumoniae | G− | 147 | 20.19 |
| Moraxella catarrhalis | G− | 75 | 10.30 |
| Escherichia coli | G− | 44 | 6.04 |
| Pseudomonas aeruginosa | G− | 29 | 3.98 |
| Enterobacter cloacae | G− | 23 | 3.16 |
| Acinetobacter baumannii | G− | 15 | 2.06 |
| Enterobacter aerogenes | G− | 10 | 1.37 |
| Stenotrophomonas maltophilia | G− | 9 | 1.24 |
| Haemophilus parainfluenzae | G− | 7 | 0.96 |
| Streptococcus pneumoniae | G+ | 70 | 9.62 |
| Staphylococcus aureus | G+ | 42 | 5.77 |
| Streptococcus viridans | G+ | 23 | 3.16 |
| Staphylococcus epidermidis | G+ | 18 | 2.47 |
| Enterococcus faecium | G+ | 6 | 0.82 |
| Total | – | 728 | 100 |
G−, Gram-negative; G+, Gram-positive.
Variable selection
Univariate logistic regression analysis identified multiple variables significantly associated with bacterial co-infection in children with influenza virus pneumonia, including younger age, higher body temperature, elevated WBC, CRP, PLT, PCT, LDH levels, presence of underlying diseases and gasping symptoms (all P<0.001). These variables were further included in the multivariate logistic regression model (Table 3).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | Z | P | OR (95% CI) | β | SE | Z | P | OR (95% CI) | ||
| Underlying diseases | |||||||||||
| No | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | |||||||||
| Yes | 1.03 | 0.12 | 8.33 | <0.001* | 2.80 (2.20–3.57) | 0.69 | 0.26 | 2.65 | 0.008* | 2.00 (1.20–3.34) | |
| Gasping | |||||||||||
| No | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | |||||||||
| Yes | 0.55 | 0.11 | 4.98 | <0.001* | 1.72 (1.39–2.14) | 0.47 | 0.23 | 2.05 | 0.040 | 1.60 (1.02–2.52) | |
| Age | −0.37 | 0.03 | −12.28 | <0.001* | 0.69 (0.65–0.74) | −0.34 | 0.05 | −6.21 | <0.001* | 0.71 (0.64–0.79) | |
| Temperature | 1.31 | 0.10 | 12.84 | <0.001* | 3.71 (3.04–4.54) | 1.37 | 0.21 | 6.53 | <0.001* | 3.94 (2.61–5.94) | |
| WBC | 0.21 | 0.02 | 11.44 | <0.001* | 1.24 (1.19–1.29) | 0.21 | 0.04 | 5.71 | <0.001* | 1.23 (1.15–1.33) | |
| CRP | 0.55 | 0.03 | 19.22 | <0.001* | 1.74 (1.65–1.84) | 0.59 | 0.04 | 13.91 | <0.001* | 1.80 (1.66–1.96) | |
| PLT | 0.01 | 0.00 | 11.29 | <0.001* | 1.01 (1.01–1.01) | 0.01 | 0.00 | 5.60 | <0.001* | 1.01 (1.01–1.01) | |
| PCT | 3.10 | 0.18 | 17.56 | <0.001* | 22.11 (15.65–31.24) | 3.66 | 0.34 | 10.62 | <0.001* | 38.80 (19.75–76.20) | |
| LDH | 0.01 | 0.00 | 4.56 | <0.001* | 1.01 (1.01–1.01) | −0.01 | 0.00 | −2.28 | 0.02* | 0.99 (0.99–0.99) | |
* indicates P<0.05, denoting statistical significance. CI, confidence interval; CRP, C-reactive protein; LDH, lactate dehydrogenase; OR, odds ratio; PCT, procalcitonin; PLT, platelet count; SE, standard error; WBC, white blood cell count.
Multivariate analysis revealed that the following variables remained independent predictors of bacterial co-infection: younger age (OR =0.71, 95% CI: 0.64–0.79, P<0.001), higher body temperature (OR =3.94, 95% CI: 2.61–5.94, P<0.001), increased WBC (OR =1.23, 95% CI: 1.15–1.33, P<0.001), CRP (OR =1.80, 95% CI: 1.66–1.96, P<0.001), PLT (OR =1.01, 95% CI: 1.01–1.01, P<0.001), and PCT (OR =38.80, 95% CI: 19.75–76.20, P<0.001). In addition, underlying diseases (OR =2.00, 95% CI: 1.20–3.34, P=0.008), gasping (OR =1.60, 95% CI: 1.02–2.52, P=0.04), and LDH (OR =0.99, 95% CI: 0.99–0.99, P=0.03) were also significantly associated with bacterial co-infection. These variables were ultimately incorporated into the prediction model for further analysis.
Construction and evaluation of the predictive model
Based on the variables selected by univariate and multivariate analyses, logistic regression analysis was conducted (Table 4). A nomogram was developed to provide individualized risk prediction based on the contribution of each variable (Figure 2A). The corresponding forest plot (Figure 2B) visualizes the strength and direction of each predictor’s effect.
Table 4
| Predictor | Estimate | SE | Z | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| (Intercept) | −68.213 | 8.422 | −8.099 | <0.001 | 0.0 | 0.0 | 0.0 |
| Age | −0.34 | 0.055 | −6.214 | <0.001 | 0.712 | 0.637 | 0.79 |
| Temperature | 1.37 | 0.21 | 6.527 | <0.001 | 3.937 | 2.632 | 6.001 |
| WBC | 0.211 | 0.037 | 5.712 | <0.001 | 1.235 | 1.15 | 1.33 |
| CRP | 0.588 | 0.042 | 13.906 | <0.001 | 1.801 | 1.666 | 1.967 |
| PLT | 0.007 | 0.001 | 5.597 | <0.001 | 1.007 | 1.005 | 1.01 |
| PCT | 3.658 | 0.344 | 10.623 | <0.001 | 38.8 | 20.242 | 78.259 |
| LDH | −0.003 | 0.001 | −2.281 | 0.02 | 0.997 | 0.995 | 1.0 |
| Underlying diseases 1 | 0.693 | 0.262 | 2.646 | 0.008 | 1.999 | 1.193 | 3.336 |
| Gasping 1 | 0.472 | 0.23 | 2.049 | 0.04 | 1.604 | 1.019 | 2.518 |
“Underlying disease 1” indicates the presence of underlying diseases. “Gasping 1” indicates the presence of gasping. CI, confidence interval; CRP, C-reactive protein; LDH, lactate dehydrogenase; OR, odds ratio; PCT, procalcitonin; PLT, platelet count; SE, standard error; WBC, white blood cell count.
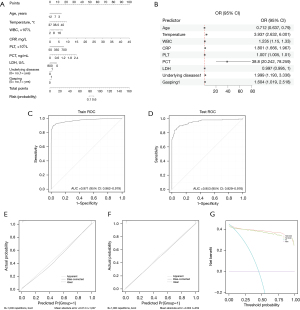
Model performance was evaluated using both training and external validation datasets. The model showed excellent discriminative ability with an AUC of 0.971 in the training set and 0.953 in the test set (Figure 2C,2D). The 5-fold cross-validation yielded a mean AUC of 0.964 (95% CI: 0.957–0.971), a mean Brier score of 0.058, and an average calibration slope of 0.98, indicating minimal overfitting. Bootstrap optimism correction produced an optimism-adjusted AUC of 0.962 and a corrected Brier score of 0.060, closely matching our original training-set performance (AUC 0.971; Brier score 0.053) and external validation (AUC 0.953; Brier score 0.098). These findings demonstrate that the model’s discriminative ability and calibration are stable across different internal splits.
Calibration curves (Figure 2E,2F) showed good agreement between predicted and observed probabilities, with Brier scores of 0.053 and 0.098 in the training and test sets, respectively. The Hosmer-Lemeshow goodness-of-fit test indicated acceptable model fit (χ2 =36.134, P<0.001). Additionally, DCA (Figure 2G) confirmed the clinical utility of the model across a range of threshold probabilities.
Overall, the nomogram enables clinicians to input patient-specific values for each predictor to obtain a total risk score, which corresponds to the predicted probability of bacterial co-infection. This facilitates individualized risk assessment and timely clinical intervention.
Discussion (V体育官网入口)
Bacterial co-infection in pediatric influenza virus pneumonia is a critical factor influencing disease progression and prognosis. A study has shown that bacterial co-infection significantly increases the length of hospital stay, the need for intensive care, and even mortality (15). Therefore, early identification of bacterial co-infection during influenza virus infection is of great significance for guiding antibiotic use, optimizing treatment strategies, and improving clinical efficiency. Currently, the diagnosis of bacterial co-infection largely depends on empirical judgment and nonspecific laboratory indicators, lacking a scientific, systematic, and accurate predictive tool. This study, based on a large dataset, developed a clinically applicable prediction model. The model was rigorously validated through multiple statistical methods and has the potential to provide precise risk stratification and optimal intervention timing for children with respiratory infections, ultimately improving outcomes.
A total of 1,966 hospitalized children with confirmed influenza virus pneumonia were included in this study. Based on the presence or absence of bacterial co-infection, patients were divided into two groups. Through comparison of clinical characteristics and multivariate logistic regression analysis, nine independent predictors were identified: age, body temperature, WBC, CRP, PLT, PCT, LDH, presence of underlying diseases, and gasping. The prediction model constructed using these variables demonstrated excellent discriminatory power in both training and test datasets, with AUC of 0.971 and 0.953, respectively. The Brier scores were 0.053 and 0.098, indicating good calibration. DCA further confirmed the model’s net clinical benefit across a range of risk thresholds. To facilitate clinical application, we also developed a visual nomogram that allows clinicians to assess the individual risk of bacterial co-infection and make informed treatment decisions accordingly. Previous studies on bacterial co-infection in influenza mainly focused on adult (16) or critically ill populations (17). Research specifically targeting children, especially involving systematic prediction modeling, remains limited. While a study has reported that biomarkers such as PCT, CRP, and WBC are closely related to bacterial infection (18), most were limited by small sample sizes (19), lack of multivariate adjustment, or absence of external validation, reducing their clinical utility (20). Building on these studies, our model integrates multiple easily accessible clinical variables and applies rigorous data modeling and evaluation methods. It balances simplicity and accuracy, enhancing its generalizability. Notably, the inclusion of clinical signs (e.g., gasping) and host-related factors (e.g., underlying diseases) improves the model’s ability to capture individual heterogeneity. Furthermore, the use of an independent test set reduces the risk of overfitting and supports the model’s robustness and reproducibility.
In terms of bacterial spectrum, our study found that Gram-negative bacteria predominated in co-infection cases, accounting for 78.16% of isolates, which is consistent with the findings of Wang et al. (14). Haemophilus influenzae, Klebsiella pneumoniae, and Moraxella catarrhalis were the three most commonly identified pathogens. This may be related to the anatomical characteristics of children’s airways, immature immune systems, and weaker mucociliary clearance, which make them more susceptible to colonizing respiratory bacteria (21). Additionally, some patients were infected with opportunistic pathogens such as Pseudomonas aeruginosa and Acinetobacter baumannii, highlighting the need for vigilance regarding nosocomial infections, especially in immunocompromised or critically ill children (22). Gram-positive bacteria accounted for 21.84%, mainly Streptococcus pneumoniae and Staphylococcus aureus, which remain important pathogens. Our findings are similar to those reported by Fu et al. (23), who found that the top 10 bacterial pathogens in Chinese children with primary infections included Escherichia coli, Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Streptococcus pyogenes, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Acinetobacter baumannii. However, our results differ from those of Klein et al. (24), whose meta-analysis of more than 3,000 influenza-related bacterial co-infection cases across multiple countries from 2003 to 2014 indicated a predominance of Gram-positive bacteria, with Streptococcus pneumoniae and Staphylococcus aureus being the most frequently identified. These differences suggest that bacterial spectra can vary with geography, seasonality, disease severity, and antibiotic usage patterns. This underscores the importance of localized pathogen surveillance and further supports the clinical relevance of our predictive model in guiding empiric antibiotic therapy before microbiological results become available.
Streptococcus pneumonia’s 9.6% share seems lower than classical results. Several factors likely contribute: (I) high PCV coverage: widespread use of pneumococcal conjugate vaccines in our pediatric population over the past decade has dramatically reduced invasive pneumococcal disease, shifting the balance toward non-vaccine serotypes and other pathogens; (II) antibiotic pre-treatment: many children receive empiric antibiotics before hospital admission and sample collection, which disproportionately suppresses Streptococcus pneumoniae growth in culture compared with more resistant Gram-negative organisms; (III) culturing challenges: Pneumococci are fastidious and may be underdetected in lower-respiratory specimens; our reliance on quantitative cultures (CFU thresholds) and exclusion of borderline-quality samples could further underestimate true pneumococcal involvement; (IV) local epidemiology: regional surveillance data in our area have consistently shown Gram-negative predominance in pediatric influenza pneumonia co-infections, reflecting local pathogen circulation patterns.
Even if multiplex PCR ultimately becomes the gold standard for detecting bacterial co-infections (25), our model remains highly valuable because it uses readily available and far less expensive inputs—single-timepoint PCT, WBC, vital signs, imaging findings, and clinical history—to stratify children by their pre-test probability of bacterial infection and thereby reserve both PCT assays and PCR panels for those most likely to benefit; in other words, by deploying the model at admission, clinicians can limit costly PCT measurements and multiplex PCR testing to high-risk patients—maximizing diagnostic yield, reducing unnecessary investigations, and controlling overall costs—while confidently managing low-risk cases without further expensive testing.
Although PCT is by far our single strongest predictor (OR =38.8), its use alone is neither perfectly specific nor optimally calibrated in this setting—severe influenza itself can elevate PCT, and reliance on a single marker risks both false positives and missed cases at different thresholds. In our cohort, a model based solely on PCT yielded an AUC of approximately 0.90 in external validation (data not shown), whereas the full logistic model achieved an AUC of 0.953, a lower Brier score (0.098 vs. ~0.12), and superior net benefit across clinically relevant risk thresholds on decision-curve analysis. By combining PCT with age, temperature, WBC, CRP, PLT, LDH, underlying disease, and gasping, we enhance discrimination, improve calibration, and provide more nuanced, patient-specific risk estimates—thereby justifying the continued value of a multivariable model over a lone PCT measurement.
The predictive model developed in this study demonstrated strong feasibility and applicability. All included variables are commonly tested in routine clinical practice and are easy to obtain at low cost, making the model suitable for use in primary healthcare settings and fever clinics. The nomogram allows intuitive risk assessment and helps clinicians determine the need for empirical antibiotic therapy, potentially reducing unnecessary antibiotic use. It may also aid in decisions regarding ICU transfer or escalation of care. In the future, this model could be integrated into electronic medical record systems for automated risk scoring, further enhancing clinical workflow. With the advancement of artificial intelligence in medicine, future models may incorporate imaging features, multi-omics data, and dynamic patient information to achieve more intelligent and precise infection management.
There are several limitations in this study. As a retrospective study, data completeness and accuracy may be affected by the quality of medical records, introducing potential information bias. The definition of “bacterial co-infection” relied on clinical judgment and microbiological testing, which may result in missed diagnoses or misdiagnoses in certain cases. Although an independent test set was used for validation, both datasets originated from the same institution during different time periods, which may limit the model’s generalizability. Multiplex PCR was not available for all patients; we relied on culture-based microbiology plus elevated markers (PCT, CRP, WBC) to define bacterial co-infection. Although we performed sensitivity analyses excluding culture-negative yet marker-positive cases, low-level or fastidious pathogens may have been missed and some “no co-infection” children could remain misclassified.
Nonetheless, as a single institution study, our findings may reflect local epidemiology, referral patterns, and clinical practices—such as thresholds for obtaining cultures, antibiotic prescribing habits, and influenza vaccination coverage—that differ from other settings. The patient population’s demographic and socioeconomic characteristics may not generalize to regions with varying healthcare access or microbial prevalence. Additionally, unmeasured center specific factors (e.g., laboratory assay platforms, imaging interpretation protocols) could influence both predictor distributions and outcome ascertainment. Prospective, multicenter validation is therefore required to confirm the model’s transportability and to refine its use across diverse clinical environments. Future research should include multi-center, prospective validation in diverse populations.
Conclusions
We developed and externally validated a predictive model for bacterial co-infection in pediatric influenza virus pneumonia, based on nine routine clinical variables: age, temperature, WBC, CRP, PLT, PCT, LDH, underlying diseases, and gasping. The model showed excellent performance and was visualized as a nomogram for individualized risk assessment. Additionally, our study updates the bacterial spectrum, with Gram-negative bacteria being predominant. This model provides a practical tool for early identification and management of bacterial co-infection, supporting timely antibiotic decision-making and improving clinical outcomes.
Acknowledgments
This work was supported by Extreme Smart Analysis platform (https://www.xsmartanalysis.com/). We acknowledge that we used generative AI (OpenAI’s ChatGPT) solely to assist with English language polishing—specifically for grammar, punctuation, and overall expression. Our interaction was limited to providing manuscript text and requesting improvements in clarity and style; at no point was ChatGPT used to analyze data, interpret findings, or alter our results. All AI suggested edits were reviewed and approved by the authors, and the scientific content remains entirely unchanged.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-340/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-340/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-340/prf
Funding: The study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-340/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. According to the policies of the Ethics Committee of The Affiliated Hospital to Changchun University of Chinese Medicine, this retrospective analysis of pre-existing, fully de-identified clinical data—with no patient contact, intervention, or change in management—was considered minimal risk and did not require formal ethics approval. The requirement for individual informed consent was waived on the grounds of de-identification and impracticability of obtaining consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
"V体育官网入口" References
- Fu C, Huang Q, Zhao J, et al. Clinical characteristics and co-infection analysis of influenza a virus in pediatric respiratory infections: a study based on tNGS technology. Eur J Clin Microbiol Infect Dis 2025;44:1695-704. [Crossref] [PubMed]
- Farzi R, Pirbonyeh N, Kadivar MR, et al. Prevalence of Influenza Viruses A and B, Adenovirus, Respiratory Syncytial Virus, and Human Metapneumonia Viruses among Children with Acute Respiratory Tract Infection. Adv Virol 2024;2024:7613948. ["V体育安卓版" Crossref] [PubMed]
- Kumar V. Influenza in Children. Indian J Pediatr 2017;84:139-43. ["V体育平台登录" Crossref] [PubMed]
- Sheikh R, Shaikh N, Ahmed M, et al. Emerging age, sex, ethnoracial, and regional trends in pneumonia and influenza-related mortality among children from 1999 to 2020. Medicine (Baltimore) 2025;104:e42027. [Crossref] [PubMed]
- Kalil AC, Thomas PG. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care 2019;23:258. [Crossref] [PubMed]
- Blyth CC, Webb SA, Kok J, et al. The impact of bacterial and viral co-infection in severe influenza. Influenza Other Respir Viruses 2013;7:168-76. [Crossref] [PubMed]
- LeMessurier KS, Tiwary M, Morin NP, et al. Respiratory Barrier as a Safeguard and Regulator of Defense Against Influenza A Virus and Streptococcus pneumoniae. Front Immunol 2020;11:3. [Crossref] [PubMed]
- Gu S, Xiao W, Yu Z, et al. Single-cell RNA-seq reveals the immune response of Co-infection with streptococcus pneumoniae after influenza A virus by a lung-on-chip: The molecular structure and mechanism of tight junction protein ZO-1. Int J Biol Macromol 2025;306:141815. [VSports app下载 - Crossref] [PubMed]
- Hu C, Zhang N, Xu D, et al. Clinical presentations and diagnostic approaches of pediatric necrotizing tracheobronchitis with influenza A virus and Staphylococcus aureus co-infections. Sci Rep 2024;14:20880. [Crossref] [PubMed]
- Pavia G, Scarpa F, Ciccozzi A, et al. Changing and Evolution of Influenza Virus: Is It a Trivial Flu? Chemotherapy 2024;69:185-93. [VSports最新版本 - Crossref] [PubMed]
- McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 2014;12:252-62. [Crossref] [PubMed]
- Martin-Loeches I, J, Schultz M, Vincent JL, et al. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med 2017;43:48-58. [Crossref] [PubMed]
- Jia L, Xie J, Zhao J, et al. Mechanisms of Severe Mortality-Associated Bacterial Co-infections Following Influenza Virus Infection. Front Cell Infect Microbiol 2017;7:338. [Crossref (VSports注册入口)] [PubMed]
- Wang QY, Yuan L, Lin JY, et al. Clinical characteristics of severe influenza virus-associated pneumonia complicated with bacterial infection in children: a retrospective analysis. BMC Infect Dis 2023;23:545. ["V体育2025版" Crossref] [PubMed]
- Shah NS, Greenberg JA, McNulty MC, et al. Bacterial and viral co-infections complicating severe influenza: Incidence and impact among 507 U.S. patients, 2013-14. J Clin Virol 2016;80:12-9. [Crossref] [PubMed]
- Abelenda-Alonso G, Rombauts A, Gudiol C, et al. Influenza and Bacterial Coinfection in Adults With Community-Acquired Pneumonia Admitted to Conventional Wards: Risk Factors, Clinical Features, and Outcomes. Open Forum Infect Dis 2020;7:ofaa066. [Crossref] [PubMed]
- Martin-Loeches I, Lemiale V, Geoghegan P, et al. Influenza and associated co-infections in critically ill immunosuppressed patients. Crit Care 2019;23:152. [Crossref] [PubMed]
- Li Z, He L, Li S, et al. Combination of procalcitonin and C-reactive protein levels in the early diagnosis of bacterial co-infections in children with H1N1 influenza. Influenza Other Respir Viruses 2019;13:184-90. [Crossref] [PubMed]
- Cuquemelle E, Soulis F, Villers D, et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med 2011;37:796-800. [V体育安卓版 - Crossref] [PubMed]
- Haran JP, Beaudoin FL, Suner S, et al. C-reactive protein as predictor of bacterial infection among patients with an influenza-like illness. Am J Emerg Med 2013;31:137-44. [Crossref] [PubMed]
- Hakansson AP, Orihuela CJ, Bogaert D. Bacterial-Host Interactions: Physiology and Pathophysiology of Respiratory Infection. Physiol Rev 2018;98:781-811. [Crossref] [PubMed]
- Ayoub Moubareck C, Hammoudi Halat D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics (Basel) 2020;9:119. [Crossref] [PubMed]
- Fu P, Xu H, Jing C, et al. Bacterial Epidemiology and Antimicrobial Resistance Profiles in Children Reported by the ISPED Program in China, 2016 to 2020. Microbiol Spectr 2021;9:e0028321. [Crossref] [PubMed]
- Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses 2016;10:394-403. [Crossref] [PubMed]
- Sanya M, Mobegi VA, Osero BO, et al. Novel highly sensitive and specific multiplex real-time pcr assay for detection of Leishmania donovani and human immunodeficiency virus-1 co-infection. Diagn Microbiol Infect Dis 2025;112:116860. [Crossref] [PubMed]


