Abstract
This study aimed to investigate the role of the intrinsic cardiac nervous system in the mechanism of classical myocardial ischaemic preconditioning (IPC). Isolated perfused rat hearts were subjected to 35-min regional ischaemia and 60-min reperfusion. IPC was induced as three cycles of 5-min global ischaemia–reperfusion, and provided significant reduction in infarct size (IS/AAR = 14 ± 2% vs control IS/AAR = 48 ± 3%, p < 0. 05). Treatment with the ganglionic antagonist, hexamethonium (50 μM), blocked IPC protection (IS/AAR = 37 ± 7%, p < 0. 05 vs IPC) V体育平台登录. Moreover, the muscarinic antagonist, atropine (100 nM), also abrogated IPC-mediated protection (IS/AAR = 40 ± 3%, p < 0. 05 vs IPC). This indicates that intrinsic cardiac ganglia remain intact in the Langendorff preparation and are important in the mechanism of IPC. In a second group of experiments, coronary effluent collected following IPC, from ex vivo perfused rat hearts, provided significant cardioprotection when perfused through a naïve isolated rat heart prior to induction of regional ischaemia–reperfusion injury (IRI) (IS/ARR = 19 ± 2, p < 0. 05 vs control effluent). This protection was also abrogated by treating the naïve heart with hexamethonium, indicating the humoral trigger of IPC induces protection via an intrinsic neuronal mechanism (IS/AAR = 46 ± 5%, p < 0. 05 vs IPC effluent). In addition, a large release in ACh was observed in coronary effluent was observed following IPC (IPCeff = 0. 36 ± 0. 03 μM vs C eff = 0. 04 ± 0. 04 μM, n = 4, p < 0. 001). Interestingly, however, IPC effluent was not able to significantly protect isolated cardiomyocytes from simulated ischaemia–reperfusion injury (cell death = 45 ± 6%, p = 0. 09 vs control effluent). In conclusion, IPC involves activation of the intrinsic cardiac nervous system, leading to release of ACh in the ventricles and induction of protection via activation of muscarinic receptors.
Keywords: Myocardial infarction, Ischaemic preconditioning, Intrinsic cardiac nervous system
Introduction
Ischaemic preconditioning (IPC) is a powerful cardioprotective phenomenon, whereby brief cycles of ischaemia to a coronary bed renders it less susceptible to subsequent ischaemia-and-reperfusion-mediated infarction [31, 51]. Indeed, IPC has emerged as a highly conserved cardioprotective intervention, effective in many mammalian species via a similar mechanistic pathway (see meta-analysis [67] and recent review [29]). Although studies have reported protection by classical IPC in the setting of cardiac surgery [32], it is not practical to be used clinically in either this setting or indeed in the in the setting of acute myocardial infarction (AMI) V体育官网入口. However, the potency of cardioprotection offered by classical IPC provides a very useful tool to better understand the physiological basis of cardioprotection.
There are three interesting mechanistic traits of classical IPC: (1) there are several triggers which initiate the protective reflex; (2) a threshold exists that must be surpassed in order for protection to occur and (3) the presence of an effector signalling pathway within the cardiomyocyte is necessary. The triggers for IPC are several small molecules, released in the heart following the brief cycles of ischaemia. These include adenosine [45], opioids [14, 60, 61] and bradykinin [66]. Blocking the receptor for one of these molecules abrogates IPC; however, this can be overcome by additional cycles of brief ischaemia [21]. Thus, IPC involves release of multiple trigger molecules that, via receptor activation, converge on a common must be reached to induce cardioprotection, relating to the strength of the IPC stimulus. Protection is observed after a single ischaemic episode of 2. 5 min, but not after shorter periods [42]. Moreover, the strength of protection seems to increase with the number of cycles of IPC, such that three cycles of 5-min ischaemia affords greater protection than one [67]. Unsurprisingly, very long IPC ischaemic cycles no longer provide any cardioprotection [67]. Finally, the effector pathway, which involves activation of several well-characterised pro-survival signalling pathways within the cardiomyocyte [27, 28] and renders the cell resistant to death. Perhaps the most interesting aspect of IPC is that, despite application of the intervention prior to the index ischaemia, the majority of protection is provided against reperfusion injury. Indeed, we have demonstrated that inhibition of the RISK pathway at the point of reperfusion abrogated IPC-mediated cardioprotection [26] VSports在线直播. This necessitates a memory phase, during which the myocardium “remembers” the protective intervention prior to its employment at reperfusion. Indeed, the initial window of protection offered by IPC lasts up to 2 h prior to the index ischaemia [41, 44]. The mechanism of this apparent memory phase is as yet unclear.
Neural control of the heart is typically thought to be mediated by regions in the brainstem and spinal cord. Indeed, the autonomic ganglia that reside within the thorax and myocardium have long been thought of as monosynaptic relay stations, which serve to confer the complex processing and efferent output of the central nervous system. In fact, there exists a complex hierarchy of cardiac neural control, with sensory afferent nerves of cardiac origin found not just in central nervous system (CNS) ganglia, but also intrathoracic and intracardiac ganglia [2, 22] V体育2025版. Intrinsic cardiac ganglia are thus able to process sensory information and control efferent post-ganglionic autonomic firing within the heart, in the absence of any central modulation [1]. Moreover, a recent study revealed a heterogeneous population of intrinsic cardiac nerves, in particular local circuit neurons, which respond to a variety of stimuli and can influence cardiac function on a beat-to-beat basis without CNS influence [7]. Thus, complex neural processing occurs within the heart, not just in response to central efferent input, but also sensory afferent information from the myocardium. Whether these reflexes remain intact in the Langendorff isolated heart preparation, however, is yet to be investigated.
The massive sensory and ischaemic trauma associated with myocardial infarction (MI) induces dynamic morphological and phenotypic remodelling of the intrinsic cardiac nervous system, not limited to the infarcted region [25]. A ‘neural sensory border zone’ of infarction appears, with those afferents within the infarcted region becoming less sensitive, and those in the border and remote regions preserved or enhanced [57]. The influence of this neural remodelling is not yet clear, although it is thought to contribute to ventricular arrhythmogenesis [13] VSports. In addition, these effects occur over a period of weeks following infarction, thus the acute influence of the intrinsic cardiac nervous system on IRI is yet to be understood.
The intrinsic cardiac nervous system has recently been implicated in the cardioprotection induced by remote ischaemic conditioning and vagus nerve stimulation [8, 55] VSports app下载. Here, we present two separate studies designed to investigate the importance of intrinsic cardiac ganglia in classical IPC; the first using an isolated perfused heart preparation, the second using a model of IPC via coronary effluent transfer.
V体育2025版 - Materials and methods
Materials
Dose justification was given in detail for hexamethonium and atropine in a recent publication from the same authors [55]. Briefly, hexamethonium (Sigma-Aldrich, Missouri, USA) was employed as a neuronal nicotinic acetylcholine receptor (nAChR) antagonist, at 50 µM, to achieve specificity at nAChRs within cardiac ganglia. Atropine, a muscarinic acetylcholine receptor (mAChR) antagonist, was used at a dose of 100 nM based on its affinity to the receptor (K d = 0 V体育官网. 36 nM).
Animals
All animals received humane care in accordance with the United Kingdom (Scientific Procedures) Act of 1986. Male Sprague–Dawley (SD) rats were bred at a central animal unit in University College London and were used at a weight of 250–300 g throughout the study.
"V体育ios版" Langendorff perfused heart preparation
Rats were anaesthetised with an upper left quadrant intraperitoneal injection of sodium pentobarbitone (60 mg/kg) (Animalcare, York, UK). Hearts were quickly excised via a clamshell thoracotomy and the aorta cannulated on a Langendorff apparatus to allow for retrograde perfusion of modified Krebs–Henseleit buffer (118 mM NaCl, 25 mM NaHCO3, 11 mM d-glucose, 4.7 mM KCl, 1.22 mM MgSO4·7H2O, 1.21 mM KH2PO4 and 1.84 mM CaCl2·2H2O. The buffer was warmed to 37.5 °C and gassed with 95% O2/5% CO2 to obtain a pH of 7.35–7.45) (for detailed methods see [9]). A fluid-filled latex balloon was inserted into the left ventricle to allow for measurement of functional parameters, including heart rate (HR) and left ventricular developed pressure (LVEDP). Coronary flow rate (CFR) was recorded throughout the protocol and the temperature of the heart was maintained at 37.0 ± 0.5°C. Finally, a 3-0 Mersilk suture (Ethicon, Edinburgh, UK) was inserted through the heart to surround the left anterior descending (LAD) coronary artery. All hearts received a 35-min LAD ischaemia and 60-min reperfusion.
Study 1
Classical ischaemic preconditioning
Two separate experiments were designed to investigate intrinsic cardiac nerves in classical ischaemic conditioning (Fig. 1). The first experiment tested the involvement of intrinsic cardiac ganglia in IPC, via use of the nicotinic acetylcholine receptor (nAChR) antagonist, hexamethonium. Isolated perfused rat hearts were randomly assigned to one of the following 4 groups: (1) sham IPC, hearts received a 40-min stabilisation period; (2) control + hexamethonium (50 μM), hearts received a 10-min stabilisation period followed by 35-min perfusion with 50 μM hexamethonium. (3) IPC3, hearts received three cycles of 5-min global ischaemia with intermittent 5-min reperfusion immediately prior to index ischaemia; (4) IPC3 + hexamethonium (50μM), same as group 4, however, the hearts were treated with hexamethonium for 5 min prior to and the duration of the 3-cycle preconditioning.
Fig. 1.

Schema detailing the experimental protocols: rat hearts were subjected to 35-min LAD ischaemia and 60-min reperfusion. Preconditioning was induced by three cycles of 5-min global ischaemia–reperfusion. Study 1, hexamethonium (1a) and atropine (1b) were perfused through the heart for 5 min prior to and for the duration of the conditioning protocol. Study 2, coronary effluent was collected from isolated hearts either following IPC or control, and subsequently perfused through (2a) a naïve isolated heart and prior to index ischaemia. Again, hexamethonium was perfused through the recipient heart for 5 min prior to and the duration of effluent perfusion. Coronary effluent was used to stimulate isolated cardiomyocytes (2b) prior to hypoxia-reoxygenation injury
The second experiment examined the importance of muscarinic acetylcholine receptors (mAChR) in classical IPC, via use of the drug atropine. Hearts were randomly assigned to one of 3 groups: (1) control + atropine (100 nM), hearts received a 10-min stabilisation followed by 35-min perfusion with 100 nM atropine; (2) IPC3, hearts received three cycles of 5-min global ischaemia with intermittent 5-min reperfusion immediately prior to index ischaemia; (3) IPC3 + atropine (100 nM), same as group 2, however, hearts were perfused with atropine for 5 min prior to and the duration of the IPC protocol.
All hearts subsequently received 35-min LAD ischaemia and 60-min reperfusion. At the end of the protocol, hearts were analysed for infarct size using methods described below.
Study 2
Classical IPC with coronary effluent transfer to naïve isolated hearts
This study uses a model first pioneered by the Przyklenk laboratory [17], and has been used in several subsequent publications by different groups [11, 43]. Although it is described in the literature as more similar to remote ischaemic preconditioning, in fact it likely reflects the humoral aspect to classical preconditioning. That is, it enables one to investigate the factors released by the heart following IPC. RIC is now generally agreed to occur via a more complex neuro-humoral pathway [55].
In the first part of the experiment, coronary effluent was collected from isolated perfused donor rat hearts, randomised into one of the following two groups: (1) donor control hearts underwent 30 min of perfusion during which effluent was collected; (2) donor IPC hearts received three cycles of 5-min global ischaemia with intermittent 5-min reperfusion, during which effluent was collected.
In the second part, recipient hearts were perfused with effluent (from above) for 10 min, following 30 min of stabilisation, immediately prior to 35-min LAD ischaemia and 60-min reperfusion. These recipient hearts were randomised to one of four groups: (1) C eff, hearts received 10-min perfusion of donor control effluent immediately prior to ischaemia; (2) IPCeff, hearts received a matched 10-min perfusion of donor IPC effluent; (3) C eff + Hex, the same as group 1, however, hearts were perfused with hexamethonium (50 μM) for 5 min prior to and the duration of effluent perfusion; (4) IPCeff + Hex, same as group 2, however, hearts were perfused with hexamethonium for 5 min prior to and the duration of effluent perfusion. Following reperfusion, all hearts were analysed for infarct size using methods described below.
"V体育官网" Acetylcholine assay
A Choline/Acetylcholine Assay Kit (Abcam, UK) was used to measure the concentration of acetylcholine in effluent collected following IPC (3 × 5-min global ischaemia–reperfusion) or corresponding control period, as described above. The assay was carried out in accordance with the instructions provided by the manufacturer. Briefly, via the use of acetylcholinesterase, the level of free and total choline was measured in each sample, enabling an estimation of the concentration of ACh within the sample.
Classical IPC with coronary effluent transfer to naïve isolated cardiomyocytes
In order to ascertain the role of the intrinsic cardiac nerves in classical IPC we undertook a series of studies using the isolated cardiomyocyte, where nerves are not present. Isolation of adult male Sprague–Dawley rat (250–300 g) cardiomyocytes was performed using a previously described protocol [30]. Cells were plated on laminin-coated 35-mm dishes (VWR international, PA, USA) and left to stabilise for 24 h prior to use. Dishes were assigned to one of the following groups: (1) normoxia, cells were left in M119 media for the duration of the protocol; (2) vector control, cells were stimulated for 10 min with Krebs–Henseleit buffer; (3) C eff, cells were stimulated for 10 min with control effluent; (4) IPCeff, cells were stimulated for 10 min with IPC effluent; (5) NECA, cells were stimulated with the adenosine A2B receptor agonist, 5′-N-ethylcarboxamidoadenosine (NECA). Following stimulation (groups 2–5), cells were treated with hypoxic buffer (NaCl 127.8 mM, 14.8 mM KCl, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 2.2 mM, CaCl2 1 mM, Na. lactate 10 mM, gassed with 5% CO2–95% N2 to achieve pH 6.4), and placed into a sealed hypoxic chamber (Billups-Rothenberg, CA, USA) filled with 5% CO2–95% N2 gas mix. Hypoxia was continued for 3 h at 37 °C, at which point the cells were removed from the chamber and treated with normoxic buffer (glucose 10 mM, NaCl 118 mM, KCl 2.6 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 22 mM, CaCl2 1 mM, gassed with 5% CO2–95% O2 to achieve pH 7.4) to simulate reperfusion. The reoxygenation was continued for 1 h, at which point the proportion of cell death was measured via propidium iodide staining and confocal microscopy (previously described here [65]).
"VSports手机版" Infarct size assessment
Infarct size of each isolated heart in the above experiments (Study 1 and 2) was calculated using the following methods, described in detail previously [9]. Briefly, at the end of the reperfusion period, the LAD suture was re-tightened and 1 ml of 0.25% Evans blue dye was perfused through the heart in order to delineate the area-at-risk of infarction. The hearts were then frozen at −20°C before being sectioned into 5 transverse slices and stained for viable tissue by immersion in 1% triphenyl-tetrazolium chloride at 37°C for 15 min. Following fixation in 10% formalin for 24 h, the sections were digitally scanned to a computer for analysis. Analysis of infarct size (IS) as a proportion of area at risk (AAR) was calculated via planimetry using imageJ software (version 1.45, National Institutes of Health, USA).
Statistical analysis
Data groups were first analysed for normality using the Kolmogorov–Smirnov test. Statistical differences between two groups were analysed using a Student’s t test and more than two groups using a one-way analysis of variance (ANOVA) with Tukey’s multiple comparison post-test. All data are presented as mean ± standard error of the mean (SEM). Data groups were classed as significantly different with a p value less than 0.05. Notation of significance is described in figure legends. Analyses were performed using GraphPad Prism version 5 for Windows (CA, USA).
Results
V体育平台登录 - Study 1: classical ischaemic preconditioning is abrogated by hexamethonium and atropine
In our isolated perfused rat heart model we demonstrated that three cycles of IPC was effective at reducing infarct size relative to control (IS/AAR = 14 ± 2% vs control IS/AAR = 48 ± 3%, p < 0.05) (Fig. 2a). The nAChR antagonist, hexamethonium (50 μM), almost fully abrogated this cardioprotection (IS/AAR = 37 ± 7%, p > 0.05 vs control). Hexamenthonium alone did not influence infarct size (IS/AAR = 44 ± 4%).
Fig. 2.
Hexamethonium and atropine abrogate ischaemic preconditioning: a Hexamethonium abrogates preconditioning induced by both one and three cycles of IPC (n = 6–8 per group, asterisk = p < 0.05 vs control); b atropine also abrogates the protection afforded by IPC3 (n = 6 per group except IPC + atropine where n = 5, asterisk = p < 0.05 vs control + atropine). Data presented as mean ± SEM
In the second part of this study, the muscarinic antagonist, atropine, was used to investigate the pathway downstream of intrinsic ganglia. 100 nM atropine did not affect infarct size (IS/AAR = 51 ± 3%); however, it abrogated the cardioprotection induced via three cycles of IPC (IS/AAR = 40 ± 3% vs IPC = 15 ± 2%) (Fig. 2b).
Study 2: factors released following classical IPC require intrinsic cardiac nerves to induce protection
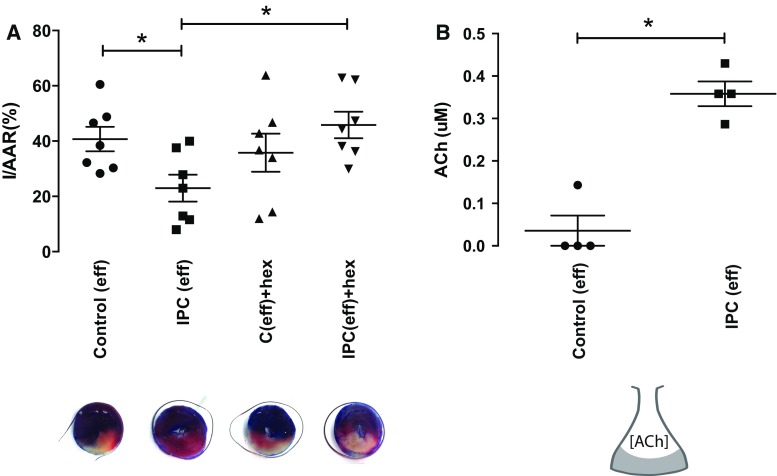
Effluent collected from hearts following classical IPC induced significant protection when perfused through a second or naïve isolated rat heart prior to acute IRI (IS/ARR = 19 ± 2, p < 0.05 vs control IS/AAR = 46 ± 6%). Pre-treatment of the naïve recipient heart with the nicotinic antagonist, hexamethonium, abrogated the protection offered by IPC effluent (IS/AAR = 46 ± 5%, p < 0.05 vs IPCeff) (Fig. 3a).
Fig. 3.
IPC effluent protects the naïve isolated heart from ischaemia–reperfusion injury. a Effluent collected following IPC significantly protected a naïve isolated rat heart via a hexamethonium-sensitive mechanism (n = 7–8 per group, asterisk = p < 0.05 vs control effluent); c the concentration of ACh in coronary effluent increases tenfold following IPC (n = 4 per group, asterisk = p < 0.05 vs Control effluent). Data presented as mean ± SEM
A large release of ACh following IPC was observed in these isolated perfused rat hearts, with a tenfold increase in the concentration relative to control effluent (IPCeff = 0.36 ± 0.03 μM vs C eff = 0.04 ± 0.04 μM, n = 4, p < 0.001) (Fig. 3b). Three of the four control effluent samples did not contain any detectable ACh.
Classical IPC effluent appears not to protect isolated cardiomyocytes from simulated IRI
Cells that were maintained under normoxic conditions throughout the experiment exhibited 27 ± 2% cell death (Fig. 4). In cells that underwent simulated IR, this was increased to 57 ± 6 and 64 ± 6% after pre-treatment with the vehicle control and control effluent, respectively (p < 0.001 vs normoxic in both cases). Treating the cells with IPC effluent did not significantly reduce cell death (45 ± 6%, p = 0.09 vs C eff). The adenosine A2b agonist NECA (used as a +ve control) significantly reduced cell death to 32 ± 4% (p < 0.01 vs vector control and C eff) (Fig. 4).
Fig. 4.
IPC effluent does not protect isolated cardiomyocytes from simulated ischaemia–reperfusion injury. a Isolated rat cardiomyocytes were not protected from hypoxia-reoxygenation injury by prior exposure to IPC effluent. The adenosine A2B agonist, NECA, was able to reduce cell death significantly (n = 6 in all groups except n = 4 for NECA, asterisk = p < 0.05 vs normoxia); b representative images of isolated cardiomyocytes subjected to the different protocols, the red staining indicate dead cells. Data presented as mean ± SEM
Discussion
These results are the first indication of a neural pathway in the mechanism of classical ischaemic preconditioning. We demonstrated an important role for nicotinic and acetylcholine receptors, suggesting that intrinsic cardiac ganglia remain active in the isolated heart preparation and are important in conveying the protective message. Acetylcholine is released from the heart following IPC, perhaps from parasympathetic post-ganglionic nerve endings in the ventricles, and induces protection via an atropine-sensitive mechanism. The lack of total abolition of protection, in the presence of ganglionic or muscarinic antagonism, is likely due to the fact that several factors contribute to the IPC mechanism in the isolated heart model [18]. Moreover, coronary effluent collected following IPC was able to protect a naïve isolated heart, but not isolated cardiomyocytes, from IRI. The protection in naïve hearts was abrogated by hexamethonium, highlighting the importance of intrinsic cardiac ganglia in the mechanism of IPC. We therefore propose that IPC is governed, in part, via a neuro-humoral pathway; a factor released following IPC activates intrinsic cardiac ganglia, leading to release of ACh from parasympathetic post-ganglionic nerve endings in the ventricles, thus inducing cardioprotection via activation of muscarinic receptors. These data are, to some extent, additive to our previous study, where we proved an important role for intrinsic cardiac ganglia in remote ischaemic conditioning. Whilst a neural pathway has been well validated in RIC, this is the first to imply a similar intrinsic cardiac neural pathway in IPC. Thus, there appear to be more similarities between the mechanisms of classical (direct) and remote ischaemic conditioning than were previously apparent [5, 55].
Intrinsic cardiac ganglia in the isolated heart
Intrinsic cardiac ganglia are widely distributed in the myocardium and not only relay central efferent pre-ganglionic information, but also are able to process sensory afferent information from the myocardium and control efferent post-ganglionic firing [7, 46]. Moreover, several anatomical and functional studies have indicated a significant presence of vagal neurons in the ventricles, in addition to sympathetic and local circuit neurons [3]. The intrinsic cardiac nervous system is therefore able to control cardiac indices on a beat-to-beat basis, in the absence of input from the central nervous system [4].
Activation of sensory afferent nerves in the Langendorff heart has previously been demonstrated to induce cardioprotection. Perfusion with capsaicin, a known activator of C-fibre afferents, induced early and delayed protection against ischaemia–reperfusion injury in isolated Langendorff rat hearts [69]. In our study, ganglionic antagonism abrogated the protection afforded by IPC, suggesting sensory afferent activation occurs following IPC and is important in conferring the cardioprotection. We further demonstrated a tenfold increase in the ACh concentration in perfusate collected following IPC. This suggests activation of post-ganglionic parasympathetic neurones from the intrinsic ganglia. Indeed, muscarinic antagonism, using atropine, abolished IPC-induced cardioprotection. The parasympathetic nervous system has a well-defined cardioprotective effect [34, 48, 49, 62], and recently emerged as a key mediator of the cardioprotection afforded by “remote” ischaemic conditioning (RIC) [6, 49, 55]. Indeed, a recent study demonstrated that increased parasympathetic tone ameliorated the functional and structural remodelling of the intrinsic cardiac nervous system following myocardial infarction [8]. Thus, we propose that there exist similarities between the mechanism of remote and classical ischaemic conditioning [37, 54]; an intrinsic neural reflex loop in response to the brief ischaemia of IPC, which activates cardiac ganglia and increases post-ganglionic vagal tone, leading to release of ACh in the ventricles.
A contentious role for acetylcholine in IPC
IPC is triggered via release of several small molecules, and their subsequent receptor activation in the myocardium. This was first demonstrated by Liu et al., who showed that pre-treatment with an adenosine receptor antagonist abrogated IPC in rabbit hearts [45]. Moreover, perfusion of exogenous adenosine through the heart prior to infarction mimicked the cardioprotection of IPC. These data suggested endogenous release of adenosine occurs in response to IPC, which protects the myocardium. Two other small molecules, opioids [14, 47, 60] and bradykinin [66], were found in subsequent studies to be important in the mechanism of IPC, via activation of their receptor. These appear to be connected via a common intracellular cytoprotective signalling pathway [53], which centres on activation of protein kinase C (PKC) [18]. Coronary effluent collected following IPC can protect a naïve isolated heart from infarction [11, 16], supporting the theory of a humoral trigger for IPC. Indeed, adenosine is released into the effluent following IPC, and confers protection to the naïve heart via crosstalk with opioid receptors [15, 43]. Bradykinin is not involved in this setting due to the requirement for circulating kininogens in the blood, not present in the isolated buffer-perfused model [21].
The role for acetylcholine in IPC, however, is more contentious. While several studies from Krieg et al. demonstrated exogenous ACh could induce cardioprotection in the Langendorff model, the same group discounts its involvement in the mechanism of IPC [38–40]. However, two studies from Kawada et al. demonstrated brief, 5-min ischaemia in in vivo rabbit and cat models induces interstitial release of ACh in the ventricles [35, 36]. Data from our study confirm those of Kawada et al. with a significant release of ACh observed in coronary effluent following IPC. Given this observation, we hypothesised that isolated cardiomyocytes would be protected from simulated IRI following exposure to coronary effluent. Presumably, if ACh was mediating protection here, it would act directly on muscarinic receptors on the cardiomyocytes in the naïve heart. However, IPC effluent was not able to significantly reduce cell death in isolated cardiomyocytes subjected to hypoxia-reoxygenation. A small reduction in cell death is observed, likely due to the presence of several trigger factors in the effluent [15, 43]; however, this was not statistically significant. This is perhaps due to dilution of factors released from the myocardium in the effluent following IPC, such that the concentration in the isolated cells would be insufficient for cardioprotection. However, we did not investigate whether exogenous ACh of the same concentration could induce cardioprotection in isolated cardiomyocytes or Langendorff models.
Finally, coronary effluent collected following IPC induced powerful cardioprotection when perfused through naïve isolated rat hearts, via a mechanism also sensitive to hexamethonium. This experiment confirms the key point to the study; factors released following IPC require intrinsic cardiac ganglia to induce cardioprotection. The neural and humoral components to IPC, therefore, are co-dependent.
Is there a common trigger for classical and remote ischaemic conditioning?
Our data suggest that both classical and remote ischaemic conditioning (RIC) may share a common trigger pathway; i.e. local release of an autocoid, activation of sensory afferent nerves and subsequent recruitment of the intrinsic cardiac nervous system. RIC is induced via the same principle as classical IPC, brief cycles of ischaemia, however applied to a region remote from the heart [10]. The trigger for RIC is thought to be local release of an autocoid, such as adenosine, which activates sensory afferent nerves communicating the protective message away from the conditioned limb. Indeed, a small injection of adenosine into the femoral artery is sufficient to induce cardioprotection [64], as is activation of C-fibre sensory afferents by capsaicin [58] or transcutaneous electrical nerve stimulation [50]. Moreover, our study has demonstrated the importance of intrinsic cardiac ganglia in IPC, which necessitates prior sensory nerve activation in the heart. Classical IPC is known to involve release of adenosine and calcitonin gene-related peptide [43, 45], both of which are know to activate sensory afferent nerves. Perhaps, therefore, these are the trigger for this aspect to IPC. The trigger for both RIC and classical IPC appear to share important similarities, with both neural and humoral components [55].
A recent meta-analysis revealed that ischaemic preconditioning had variable efficacy in mammalian species; namely, IPC was more effective in rodents relative to non-rodents [67]. Myocardial autonomic innervation is known differ according to the species’ size [59]; however, it is not clear whether these differences relate to the species-specific effect of IPC. Finally, with respect to remote ischaemic conditioning, although there appear to be differences in the signalling cascades important for cardioprotection between species [63], a recent meta-analysis revealed no difference in the efficacy of cardioprotection relative to species [12].
Finally, it is important to note the range of different factors that contribute to the trigger phase of IPC, in addition to the release of autacoids and neuro-humoral factors. Physical stimuli, such as activation of stretch receptors during the preconditioning stimulus, have been demonstrated to reduce infarct size [24]. In addition, reactive hyperaemia during brief reperfusion induces release of nitric oxide from endothelial cells, which can trigger IPC, although this effect appears to be species specific [52, 56]. Several other intracellular chemical stimuli have been implicated in the trigger phase of IPC. The brief ischaemia and reperfusion of IPC induces release of reactive oxygen species in cardiomyocytes, which in small quantities can induce cardioprotection [31]. Thus, IPC is triggered via a variety of stimuli, all of which contribute to reaching the appropriate threshold for cardioprotection to ensue.
Study limitations (V体育官网入口)
The key limitation of the study lies in the specificity of hexamethonium to neuronal nicotinic acetylcholine receptors. Hexamethonium is widely used within the literature as a ganglionic blocker, being a neuronal nicotinic acetylcholine receptor antagonist. Although it has some affinity to muscarinic M2 receptors, this becomes negligible at concentrations below 100 µM [20], and it has an IC50 of 11 µM at nAChRs [23]. Thus, for the purposes of this study 50 µM was used in order to achieve high specificity to nicotinic receptors, but the potential may remain for non-specific effects. The second issue relates to the spatial expression of nicotinic receptors within the myocardium. Although nicotinic receptors are largely limited to the intrinsic cardiac ganglia, there is evidence for expression of the receptors on the myocytes [19]. Thus, there is a small possibility that hexamethonium is exerting its effect through non-ganglionic action.
Secondly, these data do not fully ascertain the role of ACh within the effluent. An important additive experiment would be to treat a naïve isolated heart with acetylcholine at the same level observed within the effluent. In addition, it is possible that stimulation of isolated cardiomyocytes with effluent for longer than 10 min could reveal cardioprotection.
Conclusion
This is the first study to implicate intrinsic cardiac ganglia in the mechanism of classical ischaemic conditioning. We propose that IPC activates an intrinsic cardiac neural reflex and is an important part of the cardioprotective mechanism. This is a significant finding for several reasons. The Langendorff perfused heart was traditionally thought of as a denervated preparation; however, clearly this is not true given the current data, in addition to our recent publication [55]. Moreover, these data add to the paradigm that IPC is a receptor-mediated phenomenon. However, there seems to be an added layer of complexity, with the intrinsic ganglia responsible for conferring a portion of the cardioprotection. This is of importance given the issue of co-morbidities in the clinical setting. For example, diabetes has been well documented to decrease the efficacy of IPC [68]; perhaps this could be explained by the peripheral sensory neuropathy that occurs as the disease progresses [33]. Whether the peripheral sensory nerve activation induced via remote ischemic conditioning is comparable to that of classical ischaemic conditioning is not clear. Further work is necessary to ascertain the exact nature of the involvement of intrinsic ganglia in this setting.
"V体育ios版" Acknowledgements
The authors thank Prof. Alex Gourine (UCL) for his help in the initial intellectual discussions regarding intrinsic cardiac ganglia and ischaemic preconditioning.
Abbreviations
- AAR
Area-at-risk
- ACh
Acetylcholine
- AMI
Acute myocardial infarction
- CFR
Coronary flow rate
- CNS
Central nervous system
- IPC
Ischaemic preconditioning
- IRI
Ischaemia–reperfusion injury
- IS
Infarct size
- LAD
Left anterior descending
- LVEDP
Left ventricular end-diastolic pressure
- mAChR
Muscarinic acetylcholine receptor
- MI
Myocardial infarction
- nAChR
Nicotinic acetylcholine receptor
- TTC
Triphenyl tetrazolium chloride
- NECA
5′-N-Ethylcarboxamidoadenosine
References
- 1.Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. Am J Physiol. 1991;260:H713–H721. doi: 10.1152/ajpheart.1991.260.3.H713. [DOI] [PubMed] [Google Scholar]
- 2.Armour J. Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res. 1999;41:41–54. doi: 10.1016/S0008-6363(98)00252-1. ["VSports注册入口" DOI] [PubMed] [Google Scholar]
- 3.Armour JA. Potential clinical relevance of the “little brain” on the mammalian heart. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 4.Armour JA. Physiology of the intrinsic cardiac nervous system. Heart Rhythm. 2011;8:739. doi: 10.1016/j.hrthm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Basalay M, Barsukevich V, Mastitskaya S, Mrochek A, Pernow J, Sjoquist PO, Ackland GL, Gourine AV, Gourine A. Remote ischaemic pre- and delayed postconditioning—similar degree of cardioprotection but distinct mechanisms. Exp Physiol. 2012;97:908–917. doi: 10.1113/expphysiol.2012.064923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basalay MV, Mastitskaya S, Mrochek A, Ackland GL, del Arroyo AG, Sanchez J, Sjoquist P-O, Pernow J, Gourine AV, Gourine A. Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res. 2016 doi: 10.1093/cvr/cvw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol. 2013;591:4515–4533. doi: 10.1113/jphysiol.2013.259382. [DOI (V体育官网入口)] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, KenKnight BH, Armour JA, Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2015;309:H1198–H1206. doi: 10.1152/ajpheart.00393.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50:940–950. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–1646. doi: 10.1161/01.CIR.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 11.Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135–145. doi: 10.1007/s00395-010-0133-0. [VSports最新版本 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bromage DI, Pickard JM, Rossello X, Ziff OJ, Burke N, Yellon DM, Davidson SM. Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: a systematic review and meta-analysis. Cardiovasc Res. 2016 doi: 10.1093/cvr/cvw219. [VSports app下载 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen P-S, Chen LS, Fishbein MC, Lin S-F, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MV, Yang XM, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial K(ATP) channels. Circ Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. ["VSports注册入口" DOI] [PubMed] [Google Scholar]
- 15.Dickson EW, Blehar DJ, Carraway RE, Heard SO, Steinberg G, Przyklenk K. Naloxone blocks transferred preconditioning in isolated rabbit hearts. J Mol Cell Cardiol. 2001;33:1751–1756. doi: 10.1006/jmcc.2001.1436. ["VSports手机版" DOI] [PubMed] [Google Scholar]
- 16.Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, Przyklenk K. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999;277:H2451–H2457. doi: 10.1152/ajpheart.1999.277.6.H2451. [DOI] [PubMed] [Google Scholar]
- 17.Dickson EW, Porcaro WA, Fenton RA, Heard SO, Reindhardt CP, Renzi FP, Przyklenk K. “Preconditioning at a distance” in the isolated rabbit heart. Acad Emerg Med. 2000;7:311–317. doi: 10.1111/j.1553-2712.2000.tb02228.x. [DOI] [PubMed] [Google Scholar]
- 18.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 19.Dvorakova M, Lips KS, Bruggmann D, Slavikova J, Kuncova J, Kummer W. Developmental changes in the expression of nicotinic acetylcholine receptor alpha-subunits in the rat heart. Cell Tissue Res. 2005;319:201–209. doi: 10.1007/s00441-004-1008-1. [DOI] [PubMed] [Google Scholar]
- 20.Eglen RM, Michel AD, Cornett CM, Kunysz EA, Whiting RL. The interaction of hexamethonium with muscarinic receptor subtypes in vitro. Br J Pharmacol. 1989;98:499–506. doi: 10.1111/j.1476-5381.1989.tb12623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goto M, Liu Y, Yang X-M, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995;77:611–621. doi: 10.1161/01.RES.77.3.611. [DOI] [PubMed] [Google Scholar]
- 22.Gourine A, Gourine AV. Neural mechanisms of cardioprotection. Physiology. 2014;29:133–140. doi: 10.1152/physiol.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grady SR, Meinerz NM, Cao J, Reynolds AM, Picciotto MR, Changeux JP, McIntosh JM, Marks MJ, Collins AC. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J Neurochem. 2001;76:258–268. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- 24.Gysembergh A, Margonari H, Loufoua J, Ovize A, André-Fouët X, Minaire Y, Ovize M. Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Physiol. 1998;274:H955–H964. doi: 10.1152/ajpheart.1998.274.3.H955. [DOI] [PubMed] [Google Scholar]
- 25.Hardwick JC, Ryan SE, Beaumont E, Ardell JL, Southerland EM. Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton Neurosci. 2014;181:4–12. doi: 10.1016/j.autneu.2013.10.008. [DOI (VSports)] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. ["V体育平台登录" DOI] [PubMed] [Google Scholar]
- 27.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 29.Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 30.Vander Heide RS, Rim D, Hohl CM, Ganote CE. An in vitro model of myocardial ischemia utilizing isolated adult rat myocytes. J Mol Cell Cardiol. 1990;22:165–181. doi: 10.1016/0022-2828(90)91113-L. [DOI] [PubMed] [Google Scholar]
- 31.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [V体育安卓版 - DOI] [PubMed] [Google Scholar]
- 32.Jenkins DP, Pugsley WB, Alkhulaifi AM, Kemp M, Hooper J, Yellon DM. Ischaemic preconditioning reduces troponin T release in patients undergoing coronary artery bypass surgery. Heart. 1997;77:314–318. doi: 10.1136/hrt.77.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen RV, Stottrup NB, Kristiansen SB, Botker HE. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol. 2012;107:285. doi: 10.1007/s00395-012-0285-1. [DOI] [PubMed] [Google Scholar]
- 34.Katare RG, Ando M, Kakinuma Y, Arikawa M, Handa T, Yamasaki F, Sato T. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg. 2009;137:223–231. doi: 10.1016/j.jtcvs.2008.08.020. ["VSports最新版本" DOI] [PubMed] [Google Scholar]
- 35.Kawada T, Akiyama T, Shimizu S, Kamiya A, Uemura K, Li M, Shirai M, Sugimachi M. Detection of endogenous acetylcholine release during brief ischemia in the rabbit ventricle: a possible trigger for ischemic preconditioning. Life Sci. 2009;85:597–601. doi: 10.1016/j.lfs.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Kawada T, Yamazaki T, Akiyama T, Mori H, Inagaki M, Shishido T, Takaki H, Sugimachi M, Sunagawa K. Effects of brief ischaemia on myocardial acetylcholine and noradrenaline levels in anaesthetized cats. Auton Neurosci. 2002;95:37–42. doi: 10.1016/S1566-0702(01)00352-6. [DOI (V体育官网入口)] [PubMed] [Google Scholar]
- 37.Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. 2016 doi: 10.1007/s00424-016-1922-6. [DOI] [PubMed] [Google Scholar]
- 38.Krieg T, Landsberger M, Alexeyev MF, Felix SB, Cohen MV, Downey JM. Activation of Akt is essential for acetylcholine to trigger generation of oxygen free radicals. Cardiovasc Res. 2003;58:196–202. doi: 10.1016/S0008-6363(02)00861-1. [DOI] [PubMed] [Google Scholar]
- 39.Krieg T, Qin Q, McIntosh EC, Cohen MV, Downey JM. ACh and adenosine activate PI3-kinase in rabbit hearts through transactivation of receptor tyrosine kinases. Am J Physiol Heart Circ Physiol. 2002;283:H2322–H2330. doi: 10.1152/ajpheart.00474.2002. [DOI] [PubMed] [Google Scholar]
- 40.Krieg T, Qin Q, Philipp S, Alexeyev MF, Cohen MV, Downey JM. Acetylcholine and bradykinin trigger preconditioning in the heart through a pathway that includes Akt and NOS. Am J Physiol Heart Circ Physiol. 2004;287:H2606–H2611. doi: 10.1152/ajpheart.00600.2004. [DOI] [PubMed] [Google Scholar]
- 41.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–1299. doi: 10.1161/01.RES.72.6.1293. [DOI (VSports在线直播)] [PubMed] [Google Scholar]
- 42.Lawson CS, Downey JM. Preconditioning: state of the art myocardial protection. Cardiovasc Res. 1993;27:542–550. doi: 10.1093/cvr/27.4.542. [DOI] [PubMed] [Google Scholar]
- 43.Leung CH, Wang L, Nielsen JM, Tropak MB, Fu YY, Kato H, Callahan J, Redington AN, Caldarone CA. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Ther. 2014;28:7–17. doi: 10.1007/s10557-013-6489-2. [V体育安卓版 - DOI] [PubMed] [Google Scholar]
- 44.Li YW, Whittaker P, Kloner RA. The transient nature of the effect of ischemic preconditioning on myocardial infarct size and ventricular arrhythmia. Am Heart J. 1992;123:346–353. doi: 10.1016/0002-8703(92)90645-C. [DOI] [PubMed] [Google Scholar]
- 45.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.CIR.84.1.350. [DOI] [PubMed] [Google Scholar]
- 46.Longpré J-P, Salavatian S, Beaumont E, Armour JA, Ardell JL, Jacquemet V. Measure of synchrony in the activity of intrinsic cardiac neurons. Physiol Meas. 2014;35:549–566. doi: 10.1088/0967-3334/35/4/549. [VSports手机版 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu R, Li YJ, Deng HW. Evidence for calcitonin gene-related peptide-mediated ischemic preconditioning in the rat heart. Regul Pept. 1999;82:53–57. doi: 10.1016/S0167-0115(99)00039-7. [V体育官网 - DOI] [PubMed] [Google Scholar]
- 48.Mastitskaya S, Basalay M, Hosford PS, Ramage AG, Gourine A, Gourine AV. Identifying the source of a humoral factor of remote (pre)conditioning cardioprotection. PLoS ONE. 2016;11:e0150108. doi: 10.1371/journal.pone.0150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, Kasparov S, Trapp S, Ackland GL, Gourine AV. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res. 2012;95:487–494. doi: 10.1093/cvr/cvs212. [DOI (V体育官网入口)] [PMC free article] [PubMed] [Google Scholar]
- 50.Merlocco AC, Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Manlhiot C, Li J, Redington AN. Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: in vitro validation in an animal model and first human observations. Basic Res Cardiol. 2014;109:406. doi: 10.1007/s00395-014-0406-0. [DOI] [PubMed] [Google Scholar]
- 51.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI (V体育官网)] [PubMed] [Google Scholar]
- 52.Parratt JR, Vegh A. Endothelial cells, nitric oxide and ischaemic preconditioning. Basic Res Cardiol. 1996;91:27–30. doi: 10.1007/BF00788857. [DOI] [PubMed] [Google Scholar]
- 53.Peart JN, Gross GJ. Adenosine and opioid receptor-mediated cardioprotection in the rat: evidence for cross-talk between receptors. Am J Physiol Heart Circ Physiol. 2003;285:H81–H89. doi: 10.1152/ajpheart.00985.2002. ["VSports在线直播" DOI] [PubMed] [Google Scholar]
- 54.Pickard JMJ, Bøtker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia-Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAllister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ. Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol. 2015;110:453. doi: 10.1007/s00395-014-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pickard JMJ, Davidson SM, Hausenloy DJ, Yellon DM. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res Cardiol. 2016;111:50. doi: 10.1007/s00395-016-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Post H, Schulz R, Behrends M, Gres P, Umschlag C, Heusch G. No involvement of endogenous nitric oxide in classical ischemic preconditioning in swine. J Mol Cell Cardiol. 2000;32:725–733. doi: 10.1006/jmcc.2000.1117. [VSports最新版本 - DOI] [PubMed] [Google Scholar]
- 57.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodeling of the intrinsic cardiac nervous system. J Physiol. 2015;594(2):321–341. doi: 10.1113/JP271165. ["VSports app下载" DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Dai X, Manlhiot C, Li J, Redington AN. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol. 2012;107:241. doi: 10.1007/s00395-011-0241-5. [DOI] [PubMed] [Google Scholar]
- 59.Schipke J, Mayhew TM, Mühlfeld C. Allometry of left ventricular myocardial innervation. J Anat. 2014;224:518–526. doi: 10.1111/joa.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz JE, Rose E, Yao Z, Gross GJ. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol. 1995;268:H2157–H2161. doi: 10.1152/ajpheart.1995.268.5.H2157. [DOI] [PubMed] [Google Scholar]
- 61.Schulz R, Gres P, Heusch G. Role of endogenous opioids in ischemic preconditioning but not in short-term hibernation in pigs. Am J Physiol Heart Circ Physiol. 2001;280:H2175–H2181. doi: 10.1152/ajpheart.2001.280.5.H2175. [DOI] [PubMed] [Google Scholar]
- 62.Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Kumfu S, Kumphune S, Chattipakorn S, KenKnight BH, Chattipakorn N. Vagus nerve stimulation initiated late during ischemia, but not reperfusion, exerts cardioprotection via amelioration of cardiac mitochondrial dysfunction. Heart Rhythm. 2014;11:2278–2287. doi: 10.1016/j.hrthm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res. 2015;117:279–288. doi: 10.1161/CIRCRESAHA.117.306878. [DOI] [PubMed] [Google Scholar]
- 64.Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598–H1603. doi: 10.1152/ajpheart.00396.2010. [VSports注册入口 - DOI] [PubMed] [Google Scholar]
- 65.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 66.Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994;270:681–689. [PubMed (VSports在线直播)] [Google Scholar]
- 67.Wever KE, Hooijmans CR, Riksen NP, Sterenborg TB, Sena ES, Ritskes-Hoitinga M, Warlé MC. Determinants of the efficacy of cardiac ischemic preconditioning: a systematic review and meta-analysis of animal studies. PLoS ONE. 2015;10:e0142021. doi: 10.1371/journal.pone.0142021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whittington HJ, Harding I, Stephenson CI, Bell R, Hausenloy DJ, Mocanu MM, Yellon DM. Cardioprotection in the aging, diabetic heart: the loss of protective Akt signalling. Cardiovasc Res. 2013;99:694–704. doi: 10.1093/cvr/cvt140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou FW, Li YJ, Deng HW. Early and delayed protection by capsaicin against reperfusion injury in rat hearts. Zhongguo Yao Li Xue Bao. 1999;20:912–916. [PubMed] [Google Scholar]