Expanding the use of 3D printing in congenital heart surgery
Introduction
Congenital heart diseases (CHDs) are a spectrum of structural heart defects that vary considerably in their anatomical complexity. Patients with CHD usually require interventional therapies (i. e. , surgical or transcatheter/percutaneous) for either correction or palliation to mitigate against the harmful effects of their disease. Clinicians must possess a comprehensive knowledge of disease morphology and the ability to accurately interpret two- (2D) and three-dimensional (3D) imaging modalities to make informed decisions. Over the past 25 years, 3D printing has become a disruptive technology that has changed the way patients with complex CHD are managed and the education of healthcare professionals and patients within the specialty VSports最新版本. This article focuses on the current and expanding use of 3D printing in congenital heart surgery and its future directions.
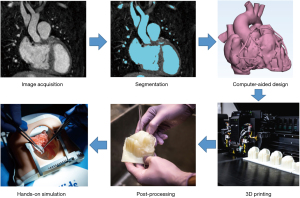
VSports注册入口 - 3D printed model workflow
The first step in the development of an accurate 3D printed heart model is the acquisition of high-quality image data. Contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) angiography are the most suitable imaging modalities for 3D model generation, owing to their high contrast resolution and spatial detail. Ideally, images should be obtained with electrocardiographic (ECG) gating and breath-holding (for CT) or respiratory navigation (for MR) (1-3) V体育平台登录. ECG-gated CT angiography is the most used modality for 3D printing and provides a spatial resolution of 0. 3–0. 7 mm. Scans should be timed for when all the cardiac chambers and great vessels are homogenously enhanced (2,3). The raw DICOM (Digital Imaging and Communication in Medicine) data is converted to the Cartesian DICOM format, which allows 3D reconstruction using voxels. This is then imported into a segmentation software (2,3). The blood pool is segmented using an automated threshold tool with manual adjustment by the user. Care must be taken if the boundary of the blood pool with the myocardium or the vessel wall is poorly defined on the image data. This is common in scans of neonates and infants, with segmentations requiring extensive manual work, which includes using drawing, erasing and regional interpolation tools to complete an accurate segmentation. This requires an excellent understanding of normal and pathologic anatomy and competency in cross-sectional image interpretation to ensure segmentation accuracy. There is now software that incorporates artificial intelligence to assist segmentation to streamline this process; however, these must be reviewed by an appropriately skilled clinician for accuracy. Once completed, the file can be exported as an STL (stereolithography) file into an appropriate CAD (computer-aided design) software, where the model can be edited to make it ready for the 3D printing process. This can include converting the blood pool into a hollow model whereby the intracardiac structures and endocardial surfaces can be demonstrated. Depending on the intended purpose of the model (i. e. , simulation), CAD representations of valves and coronary arteries can be incorporated using limited data from cross-sectional imaging and echocardiography. However, it is important to acknowledge that these additions are synthetic and may not be anatomically accurate.
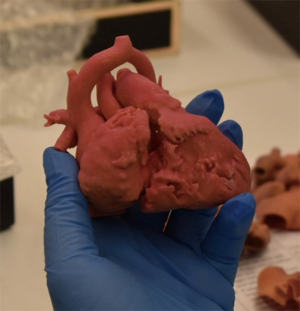
The finalised model is then ready for printing VSports注册入口. There are numerous printers available from simple, low-cost desktop printers to expensive polyjet printers, which have the capabilities to print with both soft and hard resins, depending on the model requirements and the printer capabilities. Higher-end printers [i. e. , Stratasys J750 Digital Anatomy printer (Eden Prairie, US)] provide the ability to mix materials and print in various colours and consistencies, which can be particularly useful in the demonstration of complex anatomies. The duration of the print is variable; however, for an infant-sized heart, this would be expected to take between 4–8 hours for a single model. Once printed, the model undergoes post-processing, which primarily involves removal of support material, prior to the model being ready for use (Figure 1).
Current applications
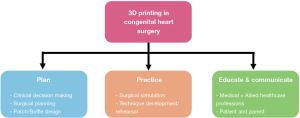
There are three main applications for the use of 3D printed models in congenital heart surgery: (I) planning (i. e. , clinical decision making, surgical planning, patch/baffle design), (II) practice (i. e. , surgical simulation, technique development/rehearsal) and (III) education (i. e. , medical/nursing, patient/parent) and communication (1,4) (Figure 2) V体育官网入口.
Surgical decision making and planning
The anatomical accuracy that can be obtained from 3D printed models has made them a valuable resource in the clinical decision-making in patients with complex forms of CHD (1,4-6). This is beneficial in borderline cases where there is ambiguity over whether a patient should have a biventricular repair or follow a univentricular pathway (1,4,5,7). Key information required for decision making includes (I) the possibility of septation of unobstructed venous inflows, (II) septation of adequately sized ventricles without compromising ventricular volume or valvar function and (III) construction of unobstructed systemic and pulmonary outflows (1,4). Conventional imaging techniques (i. e. , echocardiography, CT and MRI) provide volume and flow data that are useful in determining ventricular adequacy for septation. These can be interpreted with 3D modelling, which adds structural information to help appreciate the spatial complexity and relationships between the intracardiac structures and relationships between the heart and adjacent structures (i. e. , baffle visualisation) in addition to determining the optimal surgical approach (4,5,8) VSports在线直播.
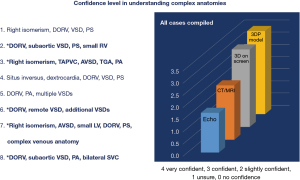
Due to the time, labour and monetary costs associated with 3D printing, consensus recommendations have been developed to guide heart teams on when a 3D model may be appropriate in decision-making (Table 1) (9). Recently, 3D printing has been included in the conventional management of patients, with procedural reimbursement codes being available in some countries (10). Cardiac morphologies where 3D printed models are useful include double outlet right ventricle (DORV), complex forms of transposition of the great arteries, heterotaxy, hearts with atrioventricular and/or ventriculoarterial discordant connections or complex ventricular septal defects (VSDs), including cases that require complex baffling (1,3,6,9). Valverde et al. demonstrated in a case-crossover study that 3D printed models were most useful in complex cases for surgical decision making. In the 40 cases that were studied, there was a change in decision-making in just under 50% of cases. There was a small but clinically significant change in the surgical approach in 9 patients and a significant change in approach (i. e. , biventricular repair vs. univentricular pathway) in 10 cases. This included 4 patients who were initially considered for conservative management who successfully underwent a biventricular repair following model review (5). Yang et al. demonstrated similar findings with 511 patients reviewed with conventional imaging by the heart team and surgical strategy being categorised into either (I) corrective, (II) equivocally corrective, (III) equivocally palliative or (IV) palliative V体育2025版. Forty patients were placed into the equivocal categories and therefore 3D printed models were made to assist decision making. In the equivocally corrective group (n=26), 23 (88%) were upgraded to corrective surgery, with 3 (12%) remaining unchanged. In the equivocally palliative group (n=14), 4 (29%) were upgraded to corrective surgery and 1 (7%) was upgraded to an equivocally corrective strategy. Of the remaining patients, 4 (26%) were confirmed for a palliative strategy, with 5 (36%) remaining in the equivocally palliative group (11). We have had the same experience in our practice with an increase in clinicians’ level of understanding complex anatomies following review of 3D-printed models (Figure 3).
"VSports" Table 1
| Usually appropriate |
| Double outlet right ventricle |
| AV and/or VA discordance (excluding single ventricle, TGA and DORV) |
| D-TGA with pulmonary stenosis |
| Heterotaxy |
| VSD with additional complexity |
| - Large VSDs extending to >1 compartment of ventricular septum |
| - Defects associated with AV valve tissue |
| - Multiple VSDs |
| - VSD with straddling tricuspid valve |
| Cases requiring complex baffling |
| MAPCA |
| Interrupted aortic arch with left ventricular outflow tract obstruction |
| Truncus arteriosus |
| Complex AVSD (unbalanced) |
| ASD with additional complexity (i.e., sinus venosus ASD with PAPVR, coronary sinus defects) |
| Maybe appropriate |
| PAPVC/TAPVC |
| Cor triatriatum |
| Single ventricle (excluding HLHS) |
| Tetralogy of Fallot |
| Aorto-pulmonary window |
| Rarely necessary |
| AVSD (balanced) |
| VSD (simple) |
| Hypoplastic left heart syndrome |
| D-TGA |
| Pulmonary vein stenosis |
| Tricuspid valve disease and Ebstein’s anomaly |
| ASD (simple) |
Adapted from Ryan JR, Ghosh R, Sturgeon G, et al. Clinical situations for which 3D printing is considered an appropriate representation or extension of data contained in a medical imaging examination: pediatric congenital heart disease conditions. 3D Print Med 2024;10:3(9) VSports. ASD, atrial septal defect; AV, atrioventricular; AVSD, atrioventricular septal defect; D-TGA, dextro-TGA; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; MAPCA, major aortopulmonary collateral arteries; PAPVC, partial anomalous pulmonary venous connection; PAPVR, partial anomalous pulmonary venous return; TAPVC, total anomalous pulmonary venous connection; TGA, transposition of the great arteries; VA, ventriculoarterial; VSD, ventricular septal defect.
Despite the benefits of 3D models in decision-making in complex CHDs users of 3D printed models must be aware of some of the limitations in the model. Firstly, at present it is extremely challenging to accurately depict the cardiac valves and subvalvular apparatus in the models; therefore, this information will be missing in the model and must be cross-referenced with echocardiography (1,3,6). Multi-modality data sets can be merged to allow integration of accurate valves into models; however, this technology is still in its infancy (12). Furthermore, for borderline ventricular septation decisions, one must be aware that the ventricular volumes presented in the model can over- or underestimate the true ventricular volume depending on what time of the cardiac cycle the images were acquired (i.e., systole vs. diastole). Therefore, such detail must be obtained when interpreting 3D printed models. We would recommend that image acquisition should occur during diastole when image quality is at its best to ensure a proper representation of the cardiac anatomy.
Baffle/patch design
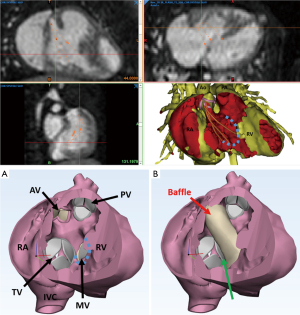
As described above, one of the most useful aspects of a 3D printed model is the ability to view if a borderline heart can be successfully septated through the creation of a baffle. Solely inspecting the model requires the surgeon to imagine how the baffle will be fashioned and the potential impact this will have on the atrioventricular valve, in addition to ensuring it will be unobstructed and not compromise ventricular cavity size. However, 3D printing technology allows the ability to graphically design the baffle and integrate this into the model (Figure 4). The workflow includes using a spline tool on the cross-sectional imaging, which allows the user to place points along the margin of the baffle during the segmentation phase and import this outline into the CAD software to fashion the patch. The heart model can be printed with the baffle in situ for the surgeon to review the feasibility for success. Alternatively, the patch can be printed as a hard cast with sterilisable material, which the surgeon can take into the operating room to assist baffle shaping with their chosen material. As the physical measurement of the printed models can be challenging and introduces potential error, we recommend that all measurements needed for clinical decision making (i.e., size of VSD, length of potential baffle) be performed on the digital segmented model. Nonetheless, studies have demonstrated that physical models are both reliable and accurate in clinical decision making (13). With the increasing availability of virtual (VR) and augmented reality (AR) there is now the possibility to develop holographically modelled templates which avoid the need and complexity of 3D printing (14).
Development of new surgical procedures
3D printed models have allowed the development of novel surgical techniques. This provides a safe environment for surgeons to plan, test, critique and refine proposed techniques prior to patient application. Successful cases include the use of partial heart transplantation of the truncal valve in truncus arteriosus and the trial of a new repair technique in addressing supravalvular aortic stenosis, both of which were tested on 3D printed models prior to performing successfully on patients (15-18). Other proposed techniques developed on 3D models include partial heart transplantation of the atrioventricular valves in complete atrioventricular septal defect (AVSD); however, this is yet to be applied to a patient due to its increased complexity compared to semilunar valve transplantation (19). Moreover, some groups are utilizing 3D-printed heart models in adult patients with failing Fontan circulation to assess the feasibility and guide planning for biventricular conversion, with the aim of halting Fontan failure and potentially avoiding future transplantation (20).
Surgical simulation
Congenital heart surgery is one of the most technically challenging surgical specialties and therefore takes a considerable length of time to master (8,21,22). Traditionally, surgeons in training were required to develop their technical competence solely by supervised surgical practice. However, since the development of 3D printed models in congenital heart surgery, modern trainees can develop their technical skills in a simulated setting (23). For over a decade the Hands-On Surgical Training (HOST) program has utilised 3D printed models to train residents and fellows across the whole spectrum of congenital heart surgery. Studies have shown that repeated practice on 3D models improves technical performance and time to complete surgical procedures and prepares surgeons for real-life operating (24-27). There are very few studies that have attempted to demonstrate a clinical benefit to patients following surgical simulation. In a small study by Li et al. demonstrated that residents with no prior experience as primary operators of VSD repair could safely perform the procedure following an intense period of simulation training of anatomically accurate 3D printed VSD models (26).
Surgeons who repeatedly practice with 3D models maintain a high level of performance over time, supporting the concept of deliberate-repeated practice. This finding was particularly apparent in more technically complex procedures such as the arterial switch operation and the Norwood operation (27,28). There has been a growth of programs utilizing 3D printed models to prepare their surgeons, demonstrating similar results, emphasizing that all trainees in congenital cardiac surgery should have hands-on simulation integrated into their training programs (29-31). Simulation with 3D printed models can help to address global workforce shortages and improve surgical outcomes in low- and middle-income countries where the provision of congenital heart surgery services is small or non-existent (32). Surgeons from these countries have limited resources to travel abroad for continued surgical education (i.e., conferences, fellowships, simulation courses). Now models can be shipped to these surgeons who can be coached by experienced surgeons during online hands-on training courses to help develop their technical skills and promote collaboration. These techniques will help facilitate global training programs, making high-quality education accessible worldwide (27,32).
Education and communication
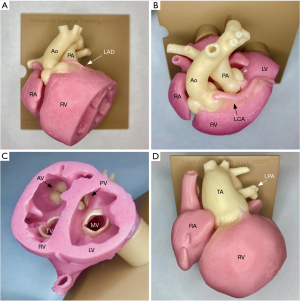
The understanding of congenital heart morphology is crucial in the management of patients with these diseases. Traditionally, this would be taught solely by using heart specimens, which are rare resources with limited durability. The use of 3D models in congenital morphology has revolutionized how modern-day clinicians and surgeons are taught (1,3,6,33,34). Unlike specimens, once the 3D model is developed, it can be printed repeatedly and can be cut in a way that can clearly demonstrate the morphology of focus (Figure 5). Alternatively, the STL file can be uploaded into a VR headset, which has been shown to be an excellent mode of morphology teaching, further expanding its reach compared to specimens (35,36). From our experience, morphology teaching is best achieved by combining specimens with 3D printed models, as there are key features in the specimen that is not replicable in the model (i.e., valvular structures) although this can be supplemented with VR (34). There are numerous studies that have demonstrated the benefits to patients and parents, and they are increasingly being used to educate and counsel prior to surgical interventions promoting shared-decision making (37-39). Although difficult to objectively quantify the value to patients and parents, this is an extremely beneficial use of incorporating 3D models into the management of patients with complex CHD. In clinical practice, 3D models facilitate rapid communication and enhance the understanding of complex anatomical relationships, particularly among team members without formal training in conventional imaging interpretation (40).
"VSports app下载" Limitations and future directions
More evidence (VSports手机版)
There are advantages to incorporating a 3D printing service within congenital heart surgery programs; however, numerous barriers exist that need to be overcome to improve its utility, quality and access. Despite emerging evidence of the perceived benefits to patients through improved pre-operative decision making, it remains unproven that using 3D printed models leads to clinically meaningful outcomes to patients (i.e., reduced operative time, morbidity and mortality). To date the evidence is derived from small studies, case reports or expert consensus documents; therefore, to achieve the desired scientific robustness would require appropriately powered prospective research including randomisation (4,8). However, it is unlikely that a clinical trial will be possible to assess the efficacy of 3D models in surgical decision making as it poses an ethical dilemma whereby patients in a control arm of a study could potentially go down an inferior and life-limiting pathway compared to if 3D models were used (5,41). It may be more appropriate to perform a retrospectively matched study; however, this produces its own challenges due to the high variability in patient morphology, particularly in the subset of patients where 3D models have been shown to be their most effective (5,6,11). With respect to simulation training, despite numerous studies showing the positive impacts in technical skill acquisition and improvement in operative efficiency over time, to date, there are only a couple of studies that have tried to demonstrate translation to real-life operating and a clinical benefit to patients (26,42). To convince training programs and insurance companies that the cost involved is justifiable, there is a need for more studies; however, it is extremely difficult to demonstrate that the 3D printed model is the reason for the outcome due to the numerous variables that contribute to this. Nonetheless, despite the paucity of high-quality scientific evidence, there are numerous qualitative reviews, editorials and expert consensus documents that strongly advise the incorporation of 3D models into the workflow of patient management and education.
Better models
Throughout the authors’ 15 years of experience with 3D printing, there have been huge advances in the quality, processes and accessibility in developing anatomically accurate models. Although print materials are still unable to replicate the mechanical characteristics of human myocardium, the use of silicone molding has substantially improved the quality of models, particularly for simulation practice compared to directly printed models (30,43) (Figure 6). The use of silicone, along with an expansion of printers available and competition, has inevitably led to cost reductions; however, this remains substantial when developing a successful 3D printing program.
Current imaging and printing technologies lack the ability to include anatomically accurate thin structures such as the atrial septum and cardiac valves. Although this may be possible with very high-resolution imaging, this is currently not widely available (35). An alternative technique is the use of multimodality imaging in generating precise 3D replicas of the heart. Rather than relying on a single imaging modality, the fusion of various imaging techniques provides a more accurate and detailed representation of the heart’s structure. This approach combines cross-sectional data from CT and MRI scans to capture the myocardium, atria, ventricles, and great arteries, while integrating valve structures from 3D echocardiography. Such advanced imaging fusion allows for more accurate and personalized models, paving the way for innovative applications in cardiology, including 3D printing (12,44).
Furthermore, we are still some time away from having very high-fidelity models for simulation that recreate the intraoperative environment and can be used to simulate full operations involving the whole surgical team rather than just the individual (45,46). This would involve developing a heart model that can be placed within a chest cavity in the correct surgical orientation with the adjacent anatomy structures in situ, which could be connected to a cardiopulmonary-bypass circuit so all stages of the operation can be simulated (i.e., cannulation and cardiac repair).
Cost effectiveness/collaboration
Congenital cardiac surgery centres will either have an in-house printing service or outsource their 3D printing to commercial companies. Costs will vary considerably depending on the model’s intended use and the production time. For clinical heart models, the cost will include both the segmentation and printing costs, whereas models used for surgical simulation or education will only require printing and post-processing costs as the segmentation and design would have already been performed previously. From our experience, a 3D printed clinical case in a flexible material would cost approximately 1,000–1,500 USD for an infant-sized model and 1,500–2,500 USD for an adult-sized heart model; however, groups have used open-source software and desktop printers get costs as low as $100 per model (47). Silicone-molded models for surgical simulation and education are approximately 275–300 USD per model; however, larger cost savings could be achieved with producing larger volumes benefiting from the economies of scale principle.
From our experience, successful in-house 3D printing programs require dedicated facilities and technical expertise, with collaboration with other medical/surgical specialities/universities to be self-sufficient and justify the capital outlay. Although out-sourcing is possible for some programs, it introduces significant cost per model, a time delay and concerns regarding sharing patient imaging/ownership of STL files; however, this should not dissuade programs from utilising such services if deemed necessary. There is an emerging need for further collaboration within the specialty to develop national and international repositories to facilitate purchasing and sharing of models (34). An alternative strategy to minimise the costs associated with 3D printing and time delay in production, is to utilize 4D endocardial surface imaging and dynamic VR rendering to produce digital models (35). This application will likely grow over time as departments become more familiar with the technology’s potential (i.e., education; understanding complex anatomy during multidisciplinary conferences); however, physical models will still be required in a subset of these patients and for hands-on simulation. Regulation remains essential for integrating emerging technologies into healthcare. Standardized guidelines for 3D models are vital for their safe and effective use (9). The FDA is committed to adapting its regulatory framework to support these innovations, prioritizing safety and efficacy while ensuring high patient care standards (10).
Conclusions
3D printing has been a transformative technology within congenital heart surgery. There has been a growth of studies that have demonstrated its benefit in decision-making in complex cases and in patients where the surgical strategy is ambiguous. Models improve the acquisition of technical skills via simulation and have been useful in the education of healthcare professionals, students and patients. Although more robust studies are needed to support its current and future applications this technology its application continues to grow. It is now paramount that institutions work together to overcome accessibility challenges and reimbursement issues.
V体育官网入口 - Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Antonio F. Corno & Ali Dodge-Khatami) for the column “Pediatric Heart” published in Translational Pediatrics. The article has undergone external peer review.
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-215/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-215/coif). The column “Pediatric Heart” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
V体育官网入口 - References
- Yoo SJ, Hussein N, Peel B, et al. 3D Modeling and Printing in Congenital Heart Surgery: Entering the Stage of Maturation. Front Pediatr 2021;9:621672. [Crossref] [PubMed]
- Yoo SJ, Lam CZ, Hussein N, et al. Chapter 3 - From Multiplanar Imaging to Physical 3D Models: 3D Printing as an Adjunct to Congenital Heart Surgery. In: Zahn EM, editor. 3-Dimensional Modeling in Cardiovascular Disease. 1st ed. Elsevier; 2020:43-54.
- Yoo SJ, Thabit O, Kim EK, et al. 3D printing in medicine of congenital heart diseases. 3D Print Med 2015;2:3.
- Illmann CF, Ghadiry-Tavi R, Hosking M, et al. Utility of 3D printed cardiac models in congenital heart disease: a scoping review. Heart 2020;106:1631-7. ["V体育官网" Crossref] [PubMed]
- Valverde I, Gomez-Ciriza G, Hussain T, et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg 2017;52:1139-48. [Crossref] [PubMed]
- Yoo SJ, van Arsdell GS. 3D Printing in Surgical Management of Double Outlet Right Ventricle. Front Pediatr 2017;5:289. [Crossref] [PubMed]
- Farooqi KM, Gonzalez-Lengua C, Shenoy R, et al. Use of a Three Dimensional Printed Cardiac Model to Assess Suitability for Biventricular Repair. World J Pediatr Congenit Heart Surg 2016;7:414-6. [Crossref (V体育官网)] [PubMed]
- Van Arsdell GS, Hussein N, Yoo SJ. Three-dimensional printing in congenital cardiac surgery-Now and the future. J Thorac Cardiovasc Surg 2020;160:515-9. [Crossref] [PubMed]
- Ryan JR, Ghosh R, Sturgeon G, et al. Clinical situations for which 3D printing is considered an appropriate representation or extension of data contained in a medical imaging examination: pediatric congenital heart disease conditions. 3D Print Med 2024;10:3.
- Ford JM, Rybicki FJ, Morris JM, et al. Stratifying complexity among the widespread use of 3D printing in United States health care facilities. 3D Print Med 2024;10:37.
- Yang DH, Park SH, Kim N, et al. Incremental Value of 3D Printing in the Preoperative Planning of Complex Congenital Heart Disease Surgery. JACC Cardiovasc Imaging 2021;14:1265-70. [Crossref] [PubMed]
- Gosnell J, Pietila T, Samuel BP, et al. Integration of Computed Tomography and Three-Dimensional Echocardiography for Hybrid Three-Dimensional Printing in Congenital Heart Disease. J Digit Imaging 2016;29:665-9. [VSports - Crossref] [PubMed]
- Shiraishi I, Yamagishi M, Hoashi T, et al. Evaluation of the Efficacy and Accuracy of Super-Flexible Three-Dimensional Heart Models of Congenital Heart Disease Made via Stereolithography Printing and Vacuum Casting: A Multicenter Clinical Trial. J Cardiovasc Dev Dis 2024;11:387. [Crossref (VSports手机版)] [PubMed]
- Lippert M, d'Albenzio G, Suther KR, et al. HoloPatch: improving intracardiac patch fit through holographically modelled templates. Eur Heart J Imaging Methods Pract 2024;2:qyae103. [Crossref] [PubMed]
- Rajab TK, Kang L, Hayden K, et al. New operations for truncus arteriosus repair using partial heart transplantation: Exploring the surgical design space with 3-dimensional printed heart models. JTCVS Tech 2023;18:91-6. [Crossref] [PubMed]
- Turek JW, Kang L, Overbey DM, et al. Partial Heart Transplant in a Neonate With Irreparable Truncal Valve Dysfunction. JAMA 2024;331:60-4. [Crossref] [PubMed]
- Hussein N, Honjo O, Barron DJ, et al. Supravalvular aortic stenosis repair: surgical training of 2 repair techniques using 3D-printed models. Interact Cardiovasc Thorac Surg 2021;33:966-8. [Crossref] [PubMed]
- Luo S, Haller C, Deng MX, et al. H-repair in supravalvular aortic stenosis. JTCVS Tech 2021;6:114-7. [Crossref] [PubMed]
- Hussein N, Turek JW, Rajab TK. Partial heart transplantation of atrioventricular valves in complete atrioventricular septal defect-simulation of techniques using silicone-molded heart models. JTCVS Tech 2023;22:251-4. [Crossref] [PubMed]
- Najm HK, Potz BA, Costello JP, et al. Biventricular Fontan conversion in criss-cross, superior-inferior ventricles using arterial switch and extra-anatomical left ventricle to aorta prosthesis. JTCVS Tech 2025;30:121-6. [Crossref] [PubMed]
- Fraser CD. Becoming a congenital heart surgeon in the current era: Realistic expectations. J Thorac Cardiovasc Surg 2016;151:1496-7. [Crossref] [PubMed]
- Fraser CD Jr, Mikulski MF, Venardos NM, et al. The journey of becoming a congenital heart surgeon: Too long, too costly, too unpredictable. J Thorac Cardiovasc Surg 2024;167:312-321.e4. [Crossref] [PubMed]
- Yoo SJ, Spray T, Austin EH 3rd, et al. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg 2017;153:1530-40. [Crossref] [PubMed]
- Hussein N, Honjo O, Haller C, et al. Quantitative assessment of technical performance during hands-on surgical training of the arterial switch operation using 3-dimensional printed heart models. J Thorac Cardiovasc Surg 2020;160:1035-42. [Crossref] [PubMed]
- Hussein N, Honjo O, Barron DJ, et al. Assessment tool validation and technical skill improvement in the simulation of the Norwood operation using three-dimensional-printed heart models. Eur J Cardiothorac Surg 2020;ezaa321. [Crossref] [PubMed]
- Li Q, Hussein N, Zhang Y, et al. Clinical translation of surgical simulated closure of a ventricular septum defect. Interact Cardiovasc Thorac Surg 2022;35:ivac122. [Crossref] [PubMed]
- Hussein N, Honjo O, Barron DJ, et al. The Incorporation of Hands-On Surgical Training in a Congenital Heart Surgery Training Curriculum. Ann Thorac Surg 2021;112:1672-80. [Crossref (V体育安卓版)] [PubMed]
- Ponzoni M, Alamri R, Peel B, et al. Longitudinal Evaluation of Congenital Cardiovascular Surgical Performance and Skills Retention Using Silicone-Molded Heart Models. World J Pediatr Congenit Heart Surg 2024;15:332-9. [Crossref] [PubMed]
- Cattapan C, Guariento A, Bertelli F, et al. The introduction of surgical simulation on three-dimensional-printed models in the cardiac surgery curriculum: an experimental project. J Cardiovasc Med (Hagerstown) 2024;25:165-72. [Crossref] [PubMed]
- Frei M, Reymond P, Wacker J, et al. Three-dimensional printed moulds to obtain silicone hearts with congenital defects for paediatric heart-surgeon training. Eur J Cardiothorac Surg 2022;65:ezae079. [Crossref] [PubMed]
- Ilina A, Lasso A, Jolley MA, et al. Patient-specific pediatric silicone heart valve models based on 3D ultrasound. Proc SPIE Int Soc Opt Eng 2017; [Crossref (VSports最新版本)]
- Deng MX, Vervoort D, Valverde I, et al. Congenital cardiac surgical simulation: bridging global workforce gaps and optimizing outcomes. Future Cardiol 2025;21:123-9. [Crossref] [PubMed]
- Loke YH, Harahsheh AS, Krieger A, et al. Usage of 3D models of tetralogy of Fallot for medical education: impact on learning congenital heart disease. BMC Med Educ 2017;17:54. [Crossref] [PubMed]
- Chetan D, Valverde I, Yoo SJ. 3D Printed Models in Cardiology Training. JACC Adv 2024;3:100893. [Crossref] [PubMed]
- Yoo SJ, Valverde I, Perens GS, et al. Four-dimensional endocardial surface imaging with dynamic virtual reality rendering: a technical note. Transl Pediatr 2024;13:1479-85. [Crossref] [PubMed]
- Brun H, Lippert M, Langø T, et al. Comparing assisting technologies for proficiency in cardiac morphology: 3D printing and mixed reality versus CT slice images for morphological understanding of congenital heart defects by medical students. Anat Sci Educ 2025;18:68-76. [Crossref (VSports)] [PubMed]
- Biglino G, Koniordou D, Gasparini M, et al. Piloting the Use of Patient-Specific Cardiac Models as a Novel Tool to Facilitate Communication During Cinical Consultations. Pediatr Cardiol 2017;38:813-8. [Crossref] [PubMed]
- Biglino G, Capelli C, Wray J, et al. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ Open 2015;5:e007165. [Crossref] [PubMed]
- Liddle D, Balsara S, Hamann K, et al. Combining patient-specific, digital 3D models with tele-education for adolescents with CHD. Cardiol Young 2022;32:912-7. [Crossref] [PubMed]
- Anwar S, Singh GK, Miller J, et al. 3D Printing is a Transformative Technology in Congenital Heart Disease. JACC Basic Transl Sci 2018;3:294-312. [Crossref] [PubMed]
- McDonald PJ, Kulkarni AV, Farrokhyar F, et al. Ethical issues in surgical research. Can J Surg 2010;53:133-6.
- Hoashi T, Ichikawa H, Nakata T, et al. Utility of a super-flexible three-dimensional printed heart model in congenital heart surgery. Interact Cardiovasc Thorac Surg 2018;27:749-55. [Crossref] [PubMed]
- Peel B, Lee W, Hussein N, et al. State-of-the-art silicone molded models for simulation of arterial switch operation: Innovation with parting-and-assembly strategy. JTCVS Tech 2022;12:132-42. ["V体育ios版" Crossref] [PubMed]
- Gomez A, Gomez G, Simpson J, et al. 3D hybrid printed models in complex congenital heart disease: 3D echocardiography and cardiovascular magnetic resonance imaging fusion. Eur Heart J 2020;41:4214. [Crossref] [PubMed]
- Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med 2006;355:2664-69. [Crossref] [PubMed]
- Stevens LM, Cooper JB, Raemer DB, et al. Educational program in crisis management for cardiac surgery teams including high realism simulation. J Thorac Cardiovasc Surg 2012;144:17-24. [Crossref] [PubMed]
- Gómez-Ciriza G, Gómez-Cía T, Rivas-González JA, et al. Affordable Three-Dimensional Printed Heart Models. Front Cardiovasc Med 2021;8:642011. [Crossref] [PubMed]