Alkaline phosphatase (ALPL) as a diagnostic and prognostic biomarker linked to immune response in thyroid cancer
"V体育2025版" Highlight box
Key findings
• Alkaline phosphatase (ALPL) is overexpressed in thyroid cancer (THCA) and promotes tumor progression and it correlates with poor prognosis and immune cell infiltration.
What is known and what is new?
• ALPL has been studied in other tumor types, but its role in THCA remains poorly defined.
• This study systematically reveals ALPL’s oncogenic function in THCA using multi-omics analysis and in vitro validation, establishing it as both a prognostic biomarker and a potential therapeutic target.
What is the implication, and what should change now?
• The identification of ALPL as a driver of tumor progression and immune modulation in THCA suggests that it may be integrated into clinical prognostic models.
• Further mechanistic studies and clinical validation are warranted to explore ALPL-targeted therapies and their role in shaping the immune microenvironment.
Introduction
Background
Thyroid cancer (THCA) is the most common endocrine malignancy globally, with its incidence rising rapidly in recent decades, posing a significant public health challenge (1,2). Between 1990 and 2020, the global prevalence of THCA increased dramatically (3). Despite its often-indolent nature, THCA can exhibit metastatic behavior in certain patients by the time of initial diagnosis. Notably, a nationwide autopsy study utilizing the Dutch pathology registry found that distant metastases were present in as many as 35 V体育官网. 1% of THCA patients, and most of these individuals harbored multiple metastatic lesions, which correlates with reduced total survival rates (4). This underscores the urgent need for reliable biomarkers to predict tumor aggressiveness and clinical outcomes. Identifying such markers would enable personalized treatment strategies, potentially improving survival rates and quality of life for patients.
Rationale and knowledge gap
Alkaline phosphatase (ALPL) encodes an enzyme critical for biological processes such as bone mineralization, lipid metabolism, and cell differentiation (5). Aberrant ALPL expression has been linked to tumor initiation, progression, and prognostic outcomes in cancer research. In breast and colorectal cancers, elevated ALPL expression correlates with increased tumor aggressiveness, metastasis, and unfavorable clinical outcomes (6). Evidence suggests that ALPL contributes to immune evasion and tumor growth by modulating immune cell infiltration and inflammatory signaling within the tumor microenvironment. Additionally, ALPL influences cancer cell differentiation, complicating therapeutic interventions in certain tumor subtypes (7). ALPL also impacts the tumor microenvironment by regulating immune cell functions, including T regulatory cells (Tregs) and macrophage polarization, thereby altering anti-tumor immune responses (8) VSports手机版. We hypothesize that ALPL has the potential to serve as a prognostic biomarker for THCA and is a promising target for therapeutic exploration.
Objective
This study aims to explore ALPL as a potential prognostic biomarker and therapeutic target, focusing on its role in tumor progression and immune infiltration.
We utilized data from The Cancer Genome Atlas (TCGA) to explore the relationship between ALPL expression and THCA V体育ios版. RNA sequencing (RNA-seq) data from TCGA were analyzed to compare ALPL expression levels in thyroid tumors versus normal tissues. We further examined the association between ALPL expression, clinical characteristics, and patient prognosis. Moreover, functional assays such as Cell Counting Kit-8 (CCK-8), colony formation, Transwell migration/invasion, 5-ethynyl-2'-deoxyuridine (EdU) staining, and flow cytometry were conducted to evaluate the effects of ALPL knockdown in THCA cell lines. Additionally, we identified a potential mechanism through which ALPL contributes to THCA progression, focusing on its role in immune infiltration. Our findings indicate that ALPL holds potential as a diagnostic/prognostic biomarker and a key therapeutic target for managing THCA. We present this article in accordance with the MDAR and REMARK reporting checklists (available at https://gs. amegroups. com/article/view/10. 21037/gs-2025-202/rc).
Methods
Data collection and analysis
The RNA-Seq expression data for ALPL across various cancers were sourced from the database of TCGA (https://portal. gdc. cancer. gov/). We used the “DESeq2” R package to recognize differentially expressed genes (DEGs) by comparing THCA samples grouped according to ALPL expression levels. The median expression value of ALPL was used as the cutoff to divide samples into high and low expression groups, a widely adopted approach in similar transcriptomic studies VSports最新版本. We screened out the genes related to the diagnosis and prognosis in THCA by screening the genes related to the prognosis of THCA and intersecting with the differential genes in THCA cancer and adjacent cancer. The analysis included the expression of ALPL levels and corresponding clinical details such as age, histological stage, and survival outcomes in THCA.
Survival analysis
Furthermore, the association between the expression of ALPL and disease-specific survival (DSS) or overall survival (OS) was analyzed across pan-cancer using TCGA data. Furthermore, correlations between ALPL expression and OS across various clinical characteristics in THCA patients were assessed using R packages V体育平台登录.
Immune characteristic analysis
The relationship between ALPL expression and immune cell infiltration was examined using the “Cibersort” R package (9). Additionally, the database of TISIDB (http://cis.hku.hk/TISIDB/) (10), a comprehensive platform for investigating tumor immune system interactions, was utilized. To further explore the immune associations of ALPL in cancer, we examined and evaluated its correlation with the expression levels of immunoregulatory genes, including immunoinhibitors, immunostimulators, chemokines, and chemokine receptors.
RNA methylation analysis
A comprehensive pan-cancer dataset, containing ALPL gene expression data and 44 marker genes linked to m1A, m5C, and m6A RNA modification pathways across various samples, was sourced from the University of California, Santa Cruz (UCSC) database (https://xenabrowser.net). Pearson correlation analysis was conducted to explore the relation between ALPL expression and the expression levels of these RNA modification marker genes.
Search Tool for the Retrieval of Interacting Genes (STRING) and Gene Multiple Association Network Integration Algorithm (GeneMANIA) database analysis
The database of STRING (www.string-db.org; doi: 10.1093/nar/gky1131) was utilized to analyze protein-protein interactions (PPIs) related to ALPL. In addition, GeneMANIA (www.genemania.org) was employed for functional gene analysis (11). Based on our study, we constructed a gene-gene interaction (GGI) network for ALPL by identifying the 20 most significant associated genes.
Functional analysis
We expanded our analysis to identify the 100 genes most strongly correlated, both positively and negatively, with ALPL expression. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were conducted to assess gene enrichment, and the results were visualized via the “ggplot2” R package. Additionally, the “GSVA” R package was applied, utilizing the single-sample gene set enrichment analysis (ssGSEA) method (12) to further enrich and enhance the analysis.
VSports最新版本 - Nomogram construction for survival prediction
In this study, independent clinicopathological prognostic factors were identified using Cox regression analysis, and a nomogram was constructed to evaluate 1-, 5-, and 10-year OS probabilities for THCA patients. To evaluate the accuracy of these predictions, calibration curves were generated to compare the predicted OS probabilities with the actual observed outcomes, presented as line charts for visual comparison.
Drug sensitivity prediction
The platform Gene Set Cancer Analysis (GSCA) (http://bioinfo.life.hust.edu.cn/GSCA/#/) offers access to data on over 750 small-molecule drugs derived from the Genomics of Cancer Therapeutics Response Portal (CTRP) (13). Leveraging this extensive resource, we analyzed the sensitivity of ALPL to small-molecule drugs using the GSCA platform.
V体育ios版 - Cell culture and transfection
The BHT101 and Cal62 THCA cell lines were bought from the Cell Bank of the Chinese Academy of Sciences (Shanghai). These cells were grown in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 U/mL penicillin-streptomycin (Pen/Strep), maintained at 37 ℃ in a humidified atmosphere with 5% CO2. ALPL small interfering RNA (siRNA) (forward 5'-GCGCAAGAGACACUGAAAUTT-3' and reverse 5'-AUUUCAGUGUCUCUUGCGCTT-3') and a negative control (NC) were obtained from GenePharma (Shanghai, China). When the BHT101 and Cal62 cells reached 80% confluence, they were seeded into six-well plates and transfected with Lipofectamine 3000 (Thermo Fisher, USA) containing the corresponding siRNA. The cells were incubated in a transfection medium with 2% FBS and 10 mg/mL polybrene for 12 hours. Then, cells were harvested 8 hours post-transfection.
"VSports最新版本" Real-time polymerase chain reaction (RT-PCR)
Samples of tumor and adjacent normal tissues were collected from 18 THCA patients for verification studies. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Sir Run Run Hospital Affiliated to Nanjing Medical University (No. 2024-SR-055). All patients signed informed consent.
SiALPL was purchased to assess knockdown efficiency in cell lines. Total RNA was converted into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA, USA) for the quantitative RT-PCR (qRT-PCR) analysis. qRT-PCR was subsequently performed on the synthesized cDNA using Power SYBR Green PCR Master Mix (Life Technologies) according to the manufacturer’s protocol. The experiments were conducted on either the Agilent Mx3005P or Applied Biosystems AB7500 Fast Real-Time PCR Systems. The primer sequences employed for qRT-PCR were as follows: ALPL (forward: 5'-ACTGGTACTCAGACAACGAGAT-3' and reverse: 5'-ACGTCAATGTCCCTGATGTTATG-3') and GAPDH (forward: 5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse: 5'-GGCTGTTGTCATACTTCTCATGG-3').
CCK-8 assay
The role of ALPL in regulating THCA cell proliferation was assessed. Following cell counting, 2,000 cells were seeded into each well of a 96-well plate, with 3–6 replicate wells per experimental group. At designated time points, CCK-8 solution, at a volume equal to 1/10th of the culture medium, was added to each well. Then the cells were incubated in a cell culture incubator for 2 hours, after which the absorbance at 450 nm was measured using a microplate reader. To assess cell viability over time, absorbance readings were taken at 0, 24, 48, 72, and 96 hours post-ALPL transfection.
Colony formation assay
After transfection, THCA cells in the exponential growth phase were counted and seeded into 6-well plates at a density of 500 cells per well. The plates were then incubated at 37 ℃ in a 5% CO2 atmosphere for 2–3 weeks to facilitate colony formation. Following incubation, the cells were rinsed twice with PBS and left to air-dry. Methanol was used to fix the cells for 15 minutes, followed by another air-drying step. The fixed cells were treated with crystal violet solution for 15 minutes, then gently rinsed with tap water to remove excess stain. Once dried, colonies were photographed using a digital camera.
Transwell migration and invasion assay
For the invasion assay, the upper surface of the Transwell inserts was pre-coated with Matrigel (BD, Franklin Lakes, NJ, USA), while this step was omitted for the migration assay. THCA cells were transfected and cultured to the exponential growth phase. The cells were enumerated and seeded into the upper compartments of the Transwell inserts at a concentration of 2,000 cells per insert, suspended in 500 µL of serum-free medium. The lower compartments of the 24-well plates were filled with 600 µL of medium enriched with 10% FBS, acting as a chemoattractant. The plates were maintained at 37 ℃ in a humidified atmosphere containing 5% CO2 for 24 hours. Following incubation, the inserts were treated with methanol for 30 minutes to fix the cells, stained with 0.1% crystal violet solution for 15 minutes, and carefully rinsed to eliminate any residual stain. Migrated or invaded cells were visualized as well as counted in six fields using by microscope.
V体育平台登录 - EdU staining
Seed THCA cells into a 6-well plate and treat them with a pre-warmed 2× EdU working solution for 2 hours. After fixing the cells, apply the Click reaction mixture to each well and incubate in the dark for 30 minutes. Finally, stain the nuclei with Hoechst and visualize the cells using a fluorescence microscope to capture images.
Flow cytometry
THCA cells were cultured in 6-well plates, and 24 hours post-transfection with ALPL siRNA, they were collected and stained using the Annexin V/7-AAD staining kit (BD) containing PI and Annexin V/7-AAD dyes. The staining process lasted for 10 minutes. Subsequently, apoptotic cells were identified and analyzed through flow cytometry.
Statistical analyses (V体育2025版)
All statistical analyses were performed using R software (version 4.2.1). Gene expression and clinical data were visualized using the “ggplot2” package. Comparisons between two groups were conducted using the unpaired Student’s t-test (for normally distributed data) or the Wilcoxon rank-sum test (for non-normally distributed data), as appropriate. For comparisons involving more than two groups, one-way analysis of variance (ANOVA) or Kruskal-Wallis test was applied.
For high-throughput analyses, such as differential gene expression and gene set enrichment analysis (GSEA), multiple hypothesis testing correction was performed using the Benjamini-Hochberg false discovery rate (FDR) method. An FDR-adjusted P value (q-value) <0.05 was considered statistically significant.
Receiver operating characteristic (ROC) curves were constructed using the “pROC” package to evaluate the diagnostic performance of ALPL and determine optimal cutoff values. Correlation analyses between ALPL expression and immune-related or methylation-related markers were assessed using Spearman’s rank correlation test. There is no missing data.
Survival analyses were carried out using the “survival” and “survminer” packages. Kaplan-Meier curves with log-rank tests were used to evaluate differences in OS between high and low ALPL expression groups. Univariate and multivariate Cox proportional hazards regression models were used to assess the prognostic value of ALPL while adjusting for relevant clinical covariates.
Statistical significance is indicated by P values below 0.05.
V体育2025版 - Results
Identification of DEGs and OS related genes in THCA
The mRNA expression profiles in THCA were analyzed for differential expression. A total of 1,747 DEGs were identified from RNA-seq-HTSeq-Counts, meeting the criteria of log2 fold change (log2FC) >1.5 and P<0.05 (Figure 1A). From TCGA data, 712 genes associated with OS in THCA were identified. A Venn diagram revealed 125 overlapping genes between THCA-related DEGs and OS-associated genes (Figure 1B).
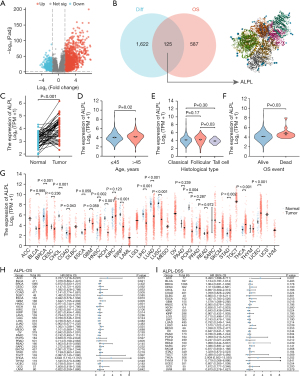
Clinical features analysis in THCA
As demonstrated in Figure 1C, the level of ALPL expression was markedly higher in THCA tissues compared to adjacent tissues (P<0.001). The association between ALPL expression and the clinical characteristics of THCA patients is summarized in Table S1. Notably, ALPL mRNA expression was significantly elevated in patients over 45 years of age compared to those aged 45 or younger (Padj=0.02) (Figure 1D). Additionally, ALPL expression was reduced in individuals with the follicular histological subtype than in those with the tall cell subtype (Padj=0.03) (Figure 1E). Moreover, ALPL mRNA levels were significantly elevated in patients who experienced OS events (dead) compared to those who did not (alive) (Padj=0.03) (Figure 1F).
Expression analysis of data in pan-cancer
In our comprehensive analysis of ALPL expression across a pan-cancer cohort from TCGA, as summarized in Table S2, ALPL expression was significantly higher in tumor tissues compared to adjacent non-tumor tissues in several cancers, including uterine corpus endometrial carcinoma (UCEC), head and neck squamous cell carcinoma (HNSC), stomach adenocarcinoma (STAD), and THCA. Conversely, reduced ALPL expression was detected in several cancer types, such as breast invasive carcinoma (BRCA), kidney renal papillary cell carcinoma (KIRP), colon adenocarcinoma (COAD), lung squamous cell carcinoma (LUSC), cholangiocarcinoma (CHOL), kidney chromophobe (KICH), lung adenocarcinoma (LUAD), liver hepatocellular carcinoma (LIHC), and pheochromocytoma and paraganglioma (PCPG) (Figure 1G).
Kaplan-Meier survival curve analysis in pan-cancer
In this study, we utilized pan-cancer data from TCGA to evaluate the impact of ALPL expression on survival outcomes by categorizing patients into high- and low-expression groups. Kaplan-Meier survival analysis revealed a significant association between elevated ALPL expression and decreased OS in several cancer types, including adrenocortical carcinoma (ACC) [hazard ratio (HR) =2.767, P=0.02], glioblastoma multiforme (GBM) (HR =1.642, P=0.005), LUSC (HR =1.362, P=0.03), and THCA (HR =3.684, P=0.03). For DSS, high ALPL expression correlated with worse outcomes in cancers such as ACC (HR =3.490, P=0.007), GBM (HR =1.579, P=0.02), and LUSC (HR =1.570, P=0.04). Conversely, lower ALPL expression was correlated with worse DSS in Kidney renal clear cell carcinoma (KIRC) (HR =0.643, P=0.02) (Figure 1H,1I).
Prognostic significance in THCA
In this study, THCA patients were classified into low- and high-ALPL expression groups to assess its effect on survival outcomes (Figure 2A). Kaplan-Meier analysis showed a significant correlation between elevated ALPL expression and poorer OS (P=0.001). Further subgroup analyses showed that high ALPL expression was consistently linked to poor outcomes across diverse demographic and clinical categories, including age >45 years (P=0.004, Figure 2B), White race (P=0.002, Figure 2C), male (P=0.03, Figure 2D) and female (P=0.02, Figure 2E) gender, pathological T3 (P=0.02, Figure 2F) and T4 (P=0.03, Figure 2G) stages, pathological N1 (P=0.004, Figure 2H), pathological M0 (P=0.003, Figure 2I), stage III (P=0.02, Figure 2J), R0 (P=0.04, Figure 2K) and R1 (P=0.005, Figure 2L), residual tumor status, presence (P=0.002, Figure 2M) or absence (P=0.02, Figure 2N) of extrathyroidal extension, and the classical histological subtype (P<0.001, Figure 2O).
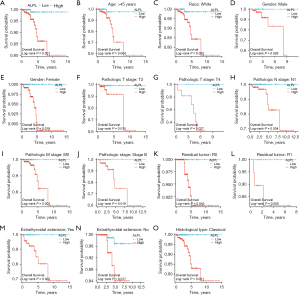
Relationship between ALPL and immunity
We created heatmaps to examine the relationship between ALPL and 22 immune cell types, aiming to better understand immune infiltration patterns across different cancers. In most cancer types, ALPL expression showed a positive correlation with M2 macrophage infiltration. However, in ACC, BRCA, acute myeloid leukemia (LAML), skin cutaneous melanoma (SKCM), and testicular germ cell tumors (TGCT), ALPL expression was negatively associated with M2 macrophages (Figure 3A). Among THCA patients, those with high ALPL expression was associated with significantly reduced infiltration of several immune cell types, including naive B cells, Tregs, resting dendritic cells (DCs), and activated DCs. In contrast, patients with low ALPL expression displayed higher infiltration levels of CD8+ T cells and M2 macrophages (Figure 3B-3H).
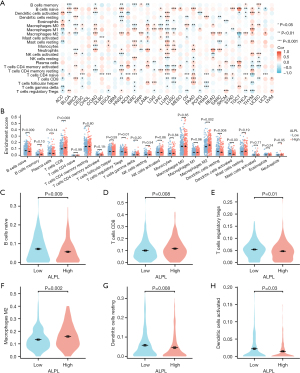
Then, we analyzed the relationship between ALPL expression and chemokine/chemokine receptor levels in THCA using TISIDB. The heatmap results revealed significant associations between the expression of ALPL showed a positive correlation with several chemokines and their respective receptors (Figure 4A,4B). Specifically, ALPL expression was positively correlated with CCL3 (r=0.163, P<0.001; Figure 4C), CXCL12 (r=0.343, P<0.001; Figure 4D), CCL14 (r=0.321, P<0.001; Figure 4E), and CCR10 (r=0.191, P<0.001; Figure 4F).
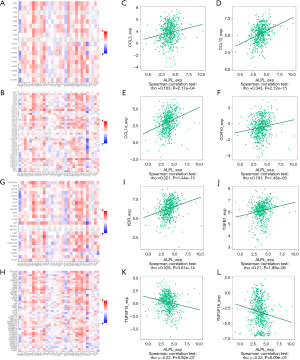
We further investigated the connection between ALPL expression and immunoinhibitory/immunostimulatory molecules in various cancers using the TISIDB database (Figure 4A,4B,4G,4H). In THCA, ALPL expression was positively related to kinase insert domain receptor (KDR) (r=0.329, P<0.001; Figure 4I), transforming growth factor beta 1 (TGFB1) (r=0.21, P<0.001; Figure 4J), and tumor necrosis factor superfamily member 15 (TNFSF15) (r=−0.220, P<0.001; Figure 4K). Conversely, ALPL expression showed a negative correlation with TNFSF18 (r=−0.220, P<0.001; Figure 4L). These results indicate that ALPL is a key factor in tumor immunity and the migration of immune cells.
Relationship between ALPL expression and methylation
The role of ALPL in influencing cancer prognosis may be partially mediated through its impact on RNA methylation. In our analysis, ALPL expression demonstrated significant positive correlations with m6A, m5C, and m1A RNA methylation regulatory genes across multiple cancer types. Specifically, in THCA, ALPL showed strong associations with the majority of m6A, m5C, and m1A regulatory genes (Figure S1). This suggests that ALPL may contribute to tumor progression by modulating the expression of RNA methylation regulators.
PPI, GGI, network, and functional enrichment analysis
A comprehensive analysis of PPI involving ALPL at various transcriptional levels was conducted using the STRING database. The resulting network, shown in Figure 5A, highlights ALPL and its interactions with 20 closely related proteins. Additionally, a GGI network for ALPL was constructed, as illustrated in Figure 5B, where the central ALPL node is connected to 20 other nodes, representing genes strongly associated with ALPL.
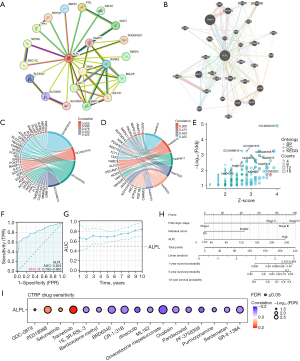
To explore the biological roles of ALPL, we conducted GO and KEGG enrichment. The results are presented in Figure 5C-5E and detailed in Table S3. Among the pathways most positively correlated with ALPL expression were the Rap1 signaling pathway (P=0.001), Fluid shear stress and atherosclerosis (P=0.001), Platelet activation (P=0.002), Endocrine resistance (P=0.003), Calcium signaling pathway (P=0.006), and vascular endothelial growth factor (VEGF) signaling pathway (P=0.009). These findings underscore ALPL’s involvement in crucial signaling and regulatory pathways, supporting its potential role in tumor progression and related pathophysiological processes.
ROC and prognostic model of ALPL in THCA
To evaluate the potential of ALPL in differentiating THCA tissue from normal tissue, ROC curve analysis revealed an area under the curve (AUC) of 0.824 (95% confidence interval: 0.783–0.865) (Figure 5F), demonstrating the strong capability of ALPL expression to distinguish between THCA and normal tissue. Additionally, the diagnostic accuracy of ALPL improved as the disease advanced in THCA patients (Figure 5G).
To further refine prognosis prediction for THCA patients, a nomogram was constructed based on Cox regression analysis (Figure 5H). Analyses were performed using univariate and multivariate methods, incorporating four key prognostic factors into the model: pathologic stage, residual tumor status, and ALPL expression (Table S4).
Drug sensitivity prediction
The relationship between ALPL expression and the sensitivity of various cancer types to pharmacological agents was assessed using the CTRP database. ALPL expression was inversely correlated with the half maximal inhibitory concentration (IC50) of drugs such as omacetaxine mepesuccinate, SR-II-138A, and CR-1-31B. Conversely, a positive correlation was observed for drugs like GDC-0879, PD318088, selumetinib, and trametinib, where higher ALPL expression corresponded to increased IC50 values (Figure 5I). These findings suggest a potential role of ALPL in influencing drug response across different cancer types.
ALPL expression validation
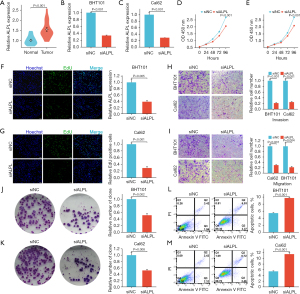
ALPL gene expression in THCA patients was further validated using RT-PCR. Results demonstrated that the expression of ALPL was notably higher in THCA tissues compared to nearby tissues (P<0.001) (Figure 6A).
VSports app下载 - ALPL promotes cell growth
The correlation between elevated ALPL expression and poor clinical outcomes highlights its potential pro-carcinogenic role in cancer. To investigate the biological functions of ALPL in THCA, siRNA was employed to knock down ALPL in BHT101 and Cal62 cell lines, with successful knockdown confirmed by RT-PCR (Figure 6B,6C). The results showed that ALPL knockdown markedly inhibited the proliferation of both BHT101 (Figure 6D,6F) and Cal62 (Figure 6E,6G) cells.
Additionally, a transwell assay revealed that ALPL knockdown significantly decreased the invasion and migration capabilities of THCA cells (Figure 6H,6I). The clonogenic potential of these cells was also significantly diminished (Figure 6J,6K). Flow cytometry further demonstrated that ALPL depletion induced a notable increase in apoptosis in THCA cells (Figure 6L,6M). These findings show that ALPL is essential for promoting tumor growth and progression in THCA.
VSports - Discussion
Key findings
Our study shows that ALPL is significantly overexpressed in THCA, particularly in patients aged over 45 years, those with tall cell histology, and those with poor survival outcomes. Kaplan-Meier survival analysis demonstrated that elevated ALPL expression correlates with significantly worse survival rates across various subgroups, including age, sex, and pathological stage. High ALPL expression was consistently associated with worse outcomes in advanced tumor-node-metastasis (TNM) stages, residual tumor status, and the presence or absence of extrathyroidal extension. These findings underscore ALPL’s value as a prognostic biomarker for THCA and its potential role in stratifying risk.
"VSports手机版" Strengths and limitations
Although this study highlights the critical role of ALPL in THCA, there are several limitations to consider. First, our analysis relied primarily on publicly available data from databases such as TCGA, and the quality and completeness of these data may impact the interpretation of the relationship between ALPL expression and clinical characteristics. Second, the study did not include laboratory validation or animal model experiments. While we observed a strong correlation between ALPL expression and immune cell infiltration, the specific molecular mechanisms underlying this relationship require further investigation. Third, although we identified associations between ALPL and the immune microenvironment, its exact role in immune evasion and tumor progression remains unclear and warrants deeper exploration. Finally, while our study proposes ALPL as a potential diagnostic and prognostic biomarker, its clinical application still needs to be confirmed through large-scale, multi-center clinical trials.
Comparison with similar research
THCA is the most common endocrine malignancy, and its incidence, especially of papillary thyroid carcinoma (PTC), has significantly increased in recent years (2). Its pathogenesis is complex, involving genetic, environmental, and immune factors (14). Although most patients have favorable outcomes, aggressive cases-especially anaplastic THCA-are characterized by high recurrence, metastasis, and poor survival rates. Current diagnostic methods, primarily based on imaging and cytology, have limitations in accurately assessing tumor invasiveness and predicting prognosis (15). This underscores the need for reliable biomarkers and targeted therapies to improve diagnostic precision, enable personalized treatment, and reduce the disease burden.
While there is limited research on ALPL’s role in tumor progression, existing studies suggest its involvement in regulating invasion in ovarian cancer (16) and inhibiting metastasis in lung adenocarcinoma (17). Additionally, ALPL downregulation has been associated with breast cancer progression to bone metastasis (6). However, its function in THCA has yet to be investigated.
Explanations of findings
Our analysis identified a notable association between ALPL expression and immune infiltration patterns. Elevated ALPL expression demonstrated a positive connection with CD8+ T cells and M2 macrophages while showing a negative correlation with naive B cells, DCs, and Tregs. M2 macrophages, known for their immunosuppressive and tumor-promoting functions, secrete cytokines like interleukin-10 (IL-10) and TGF-β, which support tumor growth and metastasis (18-20). The positive correlation between ALPL and M2 macrophages suggests that ALPL may contribute to an immunosuppressive tumor microenvironment, aiding in immune evasion.
The negative correlation between ALPL expression and B cells implies that ALPL may suppress humoral immunity, potentially diminishing antibody-mediated anti-tumor responses (21). Additionally, DCs, as critical antigen-presenting cells responsible for initiating T cell-mediated immune responses (22,23), were negatively correlated with ALPL expression. This indicates that ALPL may impair antigen presentation and T cell activation, further promoting immune evasion.
Besides, our results indicated that ALPL may promote the recruitment and activation of M2 macrophages by upregulating CCL3 expression (24). Similarly, the association with CXCL12 implies that ALPL may play a role in promoting the migration of immunosuppressive cells within the tumor microenvironment (25,26). Additionally, ALPL-induced upregulation of TGFB1 may enhance the immunosuppressive functions of M2 macrophages, contributing to tumor immune evasion (27).
Moreover, RNA methylation, as a critical epigenetic modification, controls multiple facets of RNA stability, splicing, translation, and decay, thereby affecting gene expression patterns and cellular functions (28,29). The observed correlations imply that ALPL might enhance tumorigenicity by altering the activity of key methylation regulators, potentially driving oncogenic pathways. Further investigation into the mechanistic link between ALPL and RNA methylation pathways could provide insights into its potential as a therapeutic target in THCA and other cancers.
In addition, our results highlight the significant diagnostic and prognostic value of ALPL in THCA, with ROC curve analysis showing its strong ability to differentiate THCA from normal tissues. Furthermore, a prognostic nomogram model incorporating ALPL expression, pathological stage, and residual tumor status was developed using Cox regression analysis, enhancing the prediction of patient outcomes. These findings establish ALPL as both a reliable diagnostic marker and an important prognostic indicator for THCA.
Additionally, drug sensitivity analysis further reveals the relationship between ALPL expression and responses to various anticancer drugs. Negative correlations were observed with drugs like omacetaxine mepesuccinate, indicating increased drug sensitivity at lower ALPL expression levels, while positive correlations with drugs such as selumetinib and trametinib suggest reduced sensitivity with higher ALPL expression. These insights highlight the potential role of ALPL in guiding personalized treatment strategies and optimizing therapeutic efficacy in cancer management.
Implications and actions needed
The strong correlation between ALPL expression and immune cell infiltration suggests that ALPL may play a pivotal role in shaping the tumor immune microenvironment in THCA. This finding highlights the need for further mechanistic studies to elucidate ALPL’s functional role and its interaction with immune components. Additionally, integrating ALPL expression into diagnostic and prognostic frameworks could improve early detection and inform personalized therapeutic strategies for THCA patients.
Conclusions
Our study underscores the pivotal role of ALPL in THCA, highlighting its dual function as both a diagnostic biomarker and an immunoregulatory factor. The strong correlation between ALPL expression and immune cell infiltration emphasizes its potential significance in THCA therapy. A deeper understanding of ALPL’s molecular mechanisms could improve tumor detection, inform treatment strategies, and enhance patient prognosis.
V体育平台登录 - Acknowledgments
None.
Footnote (V体育平台登录)
Reporting Checklist: The authors have completed the MDAR and REMARK reporting checklists. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-202/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-202/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-202/prf
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-202/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Sir Run Run Hospital Affiliated to Nanjing Medical University (No. 2024-SR-055). All patients signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jiang S, Huang Y, Li Y, et al. Silencing FOXP2 reverses vemurafenib resistance in BRAF(V600E) mutant papillary thyroid cancer and melanoma cells. Endocrine 2023;79:86-97. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Wild CP, Weiderpass E, Stewart BW. editors. World Cancer Report: Cancer research for cancer prevention. Lyon (FR): IARC World Cancer Reports; 2020.
- Hugen N, Sloot YJE, Netea-Maier RT, et al. Divergent Metastatic Patterns Between Subtypes of Thyroid Carcinoma Results From the Nationwide Dutch Pathology Registry. J Clin Endocrinol Metab 2020;105:e299-306. [Crossref] [PubMed]
- Jiang T, Zeng Q, He J. Do alkaline phosphatases have great potential in the diagnosis, prognosis, and treatment of tumors? Transl Cancer Res 2023;12:2932-45. [Crossref] [PubMed]
- Tayubi IA, Madar IH. Biomineralization associated alkaline phosphatase as a potential marker of bone metastasis in the patients with invasive breast cancer. Saudi J Biol Sci 2022;29:103340. [Crossref] [PubMed]
- Li H, Qian F, Bao S. Identification and functional analysis of lactic acid metabolism-related differentially expressed genes in hepatocellular carcinoma. Front Genet 2024;15:1390882. [Crossref] [PubMed]
- Li Y, You J, Zou Z, et al. Decoding the Tumor Microenvironment: Exosome-Mediated Macrophage Polarization and Therapeutic Frontiers. Int J Biol Sci 2025;21:4187-214. [Crossref] [PubMed]
- Chen B, Khodadoust MS, Liu CL, et al. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol Biol 2018;1711:243-59. [Crossref] [PubMed]
- Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284-7. [Crossref] [PubMed]
- Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 2010;38:W214-20. ["V体育ios版" Crossref] [PubMed]
- Finotello F, Trajanoski Z. Quantifying tumor-infiltrating immune cells from transcriptomics data. Cancer Immunol Immunother 2018;67:1031-40. [Crossref] [PubMed]
- Liu CJ, Hu FF, Xie GY, et al. GSCA: an integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief Bioinform 2023;24:bbac558. [Crossref] [PubMed]
- Feng X, Wang F, Yang W, et al. Association Between Genetic Risk, Adherence to Healthy Lifestyle Behavior, and Thyroid Cancer Risk. JAMA Netw Open 2022;5:e2246311. [Crossref] [PubMed]
- Chen DW, Lang BHH, McLeod DSA, et al. Thyroid cancer. Lancet 2023;401:1531-44. [Crossref] [PubMed]
- Luo M, Zhou L, Zhan SJ, et al. ALPL regulates the aggressive potential of high grade serous ovarian cancer cells via a non-canonical WNT pathway. Biochem Biophys Res Commun 2019;513:528-33. [Crossref] [PubMed]
- Lou Z, Lin W, Zhao H, et al. Alkaline phosphatase downregulation promotes lung adenocarcinoma metastasis via the c-Myc/RhoA axis. Cancer Cell Int 2021;21:217. [Crossref] [PubMed]
- Chen S, Lu K, Hou Y, et al. YY1 complex in M2 macrophage promotes prostate cancer progression by upregulating IL-6. J Immunother Cancer 2023;11:e006020. [Crossref] [PubMed]
- Wang Q, Sun Z, Guo J, et al. Tumor-derived exosomal LINC01812 induces M2 macrophage polarization to promote perineural invasion in cholangiocarcinoma. Cancer Lett 2025;617:217596. [Crossref] [PubMed]
- Qi L, Zhou B, Chen J, et al. HOXC6 promotes the metastasis of MSI-H CRC by interacting with M2 macrophages and inducing effector T cell exhaustion. Cell Commun Signal 2025;23:168. [V体育官网 - Crossref] [PubMed]
- Cells Synthesize B. Cancer Discov 2022;12:OF8. [Crossref] [PubMed]
- Wang Z, Ji X, Zhang Y, et al. Interactions between LAMP3+ dendritic cells and T-cell subpopulations promote immune evasion in papillary thyroid carcinoma. J Immunother Cancer 2024;12:e008983. [Crossref] [PubMed]
- Fu C, Jiang A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front Immunol 2018;9:3059. ["VSports手机版" Crossref] [PubMed]
- Allen F, Bobanga ID, Rauhe P, et al. CCL3 augments tumor rejection and enhances CD8+ T cell infiltration through NK and CD103+ dendritic cell recruitment via IFNγ. Oncoimmunology 2017;7:e1393598. [Crossref] [PubMed]
- Kravtsova-Ivantsiv Y, Goldhirsh G, Ciechanover A. CXCL12 restricts tumor growth by suppressing the Ras, ERK1/2, c-Myc, and the immune checkpoint PD-L1 pathways. Proc Natl Acad Sci U S A 2024;121:e2416909121. [Crossref] [PubMed]
- van der Sluis RM, García-Rodríguez JL, Nielsen IH, et al. Distinctive CD8(+) T cell activation by antigen-presenting plasmacytoid dendritic cells compared to conventional dendritic cells. Cell Rep 2025;44:115413. [Crossref] [PubMed]
- Chen Z, Ding C, Chen J, et al. Pan-cancer analysis revealing the multidimensional expression and prognostic and immunologic roles of TGFB1 in cancer. J Int Med Res 2024;52:3000605231221361. [Crossref] [PubMed]
- Han X, Wang M, Zhao YL, et al. RNA methylations in human cancers. Semin Cancer Biol 2021;75:97-115. [Crossref] [PubMed]
- Ding Y, Li X, Wang W, et al. Integrative analysis of 5-methylcytosine associated signature in papillary thyroid cancer. Sci Rep 2025;15:4405. [Crossref] [PubMed]


