Radiotherapy as a surgical alternative in ductal carcinoma in situ (DCIS): long-term survival benefits and predictors of invasive progression risk
Highlight box
Key findings
• The rising incidence of ductal carcinoma in situ (DCIS) and concerns about overtreatment in low-risk patients prompted this study. While DCIS has a favorable prognosis, up to 20–53% of patients progress to invasive breast cancer (iBCP) within 10 years. This study aimed to evaluate whether radiotherapy (RT) alone could achieve comparable survival outcomes, addressing the need for personalized management strategies for DCIS VSports在线直播.
What is known and what is new?
• Current guidelines often combine surgery with RT, but emerging evidence suggests surgery may be unnecessary for select patients.
• Using SEER data from 2,363 non-surgically treated DCIS patients, propensity score matching analysis revealed significant benefits for RT alone: 10-year breast cancer-specific survival: 99. 45% (RT) vs. 90. 50% (non-RT), P<0. 001; 10-year iBCP rate: 4. 23% (RT) vs. 13. 35% (non-RT), P<0. 001 VSports. Key predictors of RT benefit included upper-outer quadrant tumor location, cribriform histology, and hormone receptor-positive status. Cox regression identified tumor size >10 mm, high grade, and hormone receptor-negative status as independent risk factors for iBCP.
What is the implication, and what should change now?
• This study challenges the necessity of surgery for select DCIS patients, demonstrating that RT alone achieves survival rates comparable to surgical standards while halving iBCP risk V体育官网. By identifying patient subgroups that derive maximal benefit, these findings support de-escalating treatment to reduce surgical morbidity. The results provide a framework for personalized DCIS management, potentially sparing low-risk patients from overtreatment. Additionally, they inform ongoing clinical trials exploring non-surgical approaches, advancing precision oncology in early breast cancer care.
V体育官网 - Introduction
Ductal carcinoma in situ (DCIS) is a heterogeneous lesion characterized by the proliferation of ductal tumor cells confined within the breast ducts (1). Due to advancements in screening methods and the expansion of the screening scope, DCIS detection rates are steadily increasing in all countries, currently accounting for 20–25% of all breast cancers (2,3). However, the absence of declining rates of advanced breast cancer suggests overdiagnosis and overtreatment in some cases of DCIS VSports手机版.
The overall prognosis of DCIS is favorable, with only 1. 0–2. 6% of women diagnosed with DCIS dying from breast cancer within 8–10 years after diagnosis (4,5). Many DCIS lesions never progress to invasive cancer and may even show morphological signs of regression (6) V体育安卓版. However, it is important to note that there is significant heterogeneity within this disease, with 20–53% of patients biopsied and diagnosed with DCIS later being diagnosed with invasive breast cancer within 10 years or more (7,8)—a phenomenon known as invasive breast cancer progression (iBCP) (9,10). The total mortality rate within 20 years after initial diagnosis for the group experiencing iBCP is as high as 13. 5% (11). Therefore, reducing iBCP in DCIS has become an important step in extending the survival time of DCIS patients (12).
Currently, local treatment options for DCIS include breast-conserving surgery (BCS), mastectomy, and radiotherapy (RT) (13). Most DCIS patients undergo local treatment plans that combine BCS with RT. Due to the internal heterogeneity of DCIS (6,7), numerous studies have concluded that some low-risk DCIS patients may be receiving overtreatment (14).
RT, as a routine treatment plan after BCS for DCIS, has been proven by multiple studies that some DCIS patients could be exempted from RT after undergoing BCS (15,16). Additionally, four large clinical trials are currently investigating active monitoring of DCIS patients exempt from BCS [LORIS (17), COMET (18), LORD (19), LORETTA (20)]. Given the difficulty and slow progress of clinical trial recruitment, no related research results have been reported yet. Previous studies based on The Surveillance, Epidemiology, and End Results (SEER) database indicated that in DCIS patients who did not receive local treatment (surgery and RT), the ipsilateral invasive cancer incidence rate and total mortality rate within 10 years were 10.5% [95% confidence interval (CI): 8.5–12.4%], and 24.1% (95% CI: 21.2–26.9%), respectively (21). Due to advances in breast cancer screening and the diagnosis and treatment of DCIS, as well as differences in the risk of competing mortality in the population covered by the study, this result is nearly 1/3 lower compared to the iBCP reported in other retrospective clinical studies (22,23). However, this study does not explore the impact of treatments other than surgery on the prognosis of DCIS.
This study focused on the feasibility of exempting DCIS patients from BCS and offering them RT alone. We investigated the effects of RT alone on iBCP, overall survival (OS), and breast cancer-specific survival (BCSS), as well as performed subgroup analyses on the survival benefits of RT alone. Additionally, by exploring the factors affecting iBCP in DCIS patients, this study aimed to guide the individualized selection of local treatment for DCIS and provide insights for the design of future clinical trials related to the local treatment of DCIS patients. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-104/rc).
Methods
Data collection
SEER database of the National Cancer Institute (NCI) has been collecting data on the incidence, mortality, and disease conditions of cancer patients in some U.S. counties (about 35% of the U.S. population) since 1973. Using the SEER*Stat software, data on cases diagnosed with primary breast cancer from 2000 to 2018 were retrieved from the SEER database as of December 18, 2023. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Inclusion criteria: (I) diagnose time was between 2000 and 2018; (II) primary site was breast; (III) patients were older than 18 years; (IV) sequence number was “one primary” or “1st of 2 or more primaries”; (V) the initial diagnosis was DCIS; (VI) no surgery, systemic treatment, or chemotherapy. Exclusion criteria: (I) unknown tumor laterality; (II) inconsistent surgical status with the reason for surgery; (III) patients who died within 6 months of diagnosis (as death within 6 months from breast cancer suggests characteristics inconsistent with DCIS); (IV) surgery performed within 3 months of diagnosis (this short interval provides insufficient observation time and makes it difficult to distinguish baseline cancer from early new events); (V) patients diagnosed with contralateral breast cancer at the second diagnosis (this study focuses on ipsilateral progression, and progression is only considered if laterality matches). In cases where lesions were near quadrant boundaries or puncture location varied, exact quadrant matches were not required for classification (Figure 1).
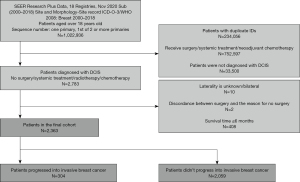
Sensitivity analysis
Some patients’ cause-specific death classification indicated death from breast cancer without progression being recorded. These patients were assumed to have progressed since death from breast cancer without progression is unlikely. A total of 304 progression cases were modified. Due to the absence of exact time to relapse, calculations were performed using interval-censored Kaplan-Meier analysis. Ultimately, 304 cases of DCIS that progressed to invasive cancer without systematic treatment and 2,059 cases of DCIS that did not progress to invasive cancer without systematic treatment (e.g., cyclophosphamide, paclitaxel, 5-fluorouracil alone or in combination, tamoxifen, and other endocrine treatments) were selected.
Statistical analysis
The relationship between baseline characteristics and outcomes and whether patients received RT alone was analyzed using chi-square tests. Differences between groups were balanced using propensity score matching (PSM), and Kaplan-Meier methods were used to draw the cumulative occurrence curves of OS, BCSS, and iBCP for the study cohort and between the RT and non-RT groups, along with subgroup analysis to find survival benefits factors of RT alone. Univariate and multivariate Cox regression analyses and competitive risk models were used to analyze factors related to the progression of DCIS to invasive cancer. All statistical analyses were performed using SPSS 29.0 and R language 4.0.4 (including packages like tableone, survey, reshape2, ggplot2, survminer, forestploter, cmprsk, etc.). A significance level of P<0.05 was considered statistically significant, and P<0.001 was considered highly significant.
Results
"V体育2025版" Clinical and pathological characteristics of enrolled patients
Table 1 summarizes the overall characteristics of the cohort. Among the total cohort of 2,363 non-surgically treated cases, 202 patients (8.55%) received local RT at the time of the first diagnosis, while 2,161 cases (91.45%) did not receive any local treatment. With certain characteristics, such as tumors ≤10 mm in diameter, high histological grade, and hormone receptor (HR)-negative were more inclined to receive RT alone. The proportion of patients receiving RT alone decreased with age (10.00–0.04%).
Table 1
| Variable | Total (n=2,363) | Radiation | χ2 | P | |
|---|---|---|---|---|---|
| No (n=2,161) | Yes (n=202) | ||||
| Year of diagnosis | 83.28 | <0.001* | |||
| 2000–2009 | 1,265 (53.53) | 1,095 (50.67) | 170 (84.16) | ||
| 2010–2018 | 1,098 (46.47) | 1,066 (49.33) | 32 (15.84) | ||
| Age (years) | 18.04 | <0.001* | |||
| 18–44 | 240 (10.16) | 216 (10.00) | 24 (11.88) | ||
| 45–55 | 673 (28.48) | 602 (27.86) | 71 (35.15) | ||
| 56–74 | 947 (40.08) | 860 (39.80) | 87 (43.07) | ||
| 75+ | 503 (21.29) | 483 (22.35) | 20 (9.90) | ||
| Race | 19.77 | <0.001* | |||
| White | 1,589 (67.25) | 1,426 (65.99) | 163 (80.69) | ||
| Black | 277 (11.72) | 260 (12.03) | 17 (8.42) | ||
| Asian or Pacific Islander | 270 (11.43) | 255 (11.80) | 15 (7.43) | ||
| Others | 227 (9.61) | 220 (10.18) | 7 (3.47) | ||
| Laterality | 1.93 | 0.17 | |||
| Left | 1,188 (50.28) | 1,077 (49.84) | 111 (54.95) | ||
| Right | 1,175 (49.72) | 1,084 (50.16) | 91 (45.05) | ||
| Primary site | 2.24 | 0.82 | |||
| Upper-outer quadrant | 615 (26.03) | 559 (25.87) | 56 (27.72) | ||
| Upper-inner quadrant | 195 (8.25) | 179 (8.28) | 16 (7.92) | ||
| Central portion & nipple | 144 (6.09) | 131 (6.06) | 13 (6.44) | ||
| Lower-inner quadrant | 132 (5.59) | 120 (5.55) | 12 (5.94) | ||
| Lower-outer quadrant | 119 (5.04) | 113 (5.23) | 6 (2.97) | ||
| Others | 1,158 (49.01) | 1,059 (49.01) | 99 (49.01) | ||
| Histology | 5.42 | 0.49 | |||
| Intraductal carcinoma | 1,397 (59.12) | 1,282 (59.32) | 115 (56.93) | ||
| Cribriform carcinoma | 315 (13.33) | 290 (13.42) | 25 (12.38) | ||
| Comedocarcinoma | 250 (10.58) | 229 (10.60) | 21 (10.40) | ||
| Solid type | 182 (7.70) | 159 (7.36) | 23 (11.39) | ||
| Papillary carcinoma | 99 (4.19) | 92 (4.26) | 7 (3.47) | ||
| Micropapillary carcinoma | 84 (3.55) | 75 (3.47) | 9 (4.46) | ||
| Others | 36 (1.52) | 34 (1.57) | 2 (0.99) | ||
| Tumor size | 6.43 | 0.04* | |||
| ≤10 mm | 268 (11.34) | 249 (11.52) | 19 (9.41) | ||
| >10 mm | 264 (11.17) | 251 (11.61) | 13 (6.44) | ||
| Unknown | 1,831 (77.49) | 1,661 (76.86) | 170 (84.16) | ||
| Grade | 1.30 | 0.52 | |||
| Grade I/II | 1,012 (42.83) | 933 (43.17) | 79 (39.11) | ||
| Grade III/IV | 683 (28.90) | 622 (28.78) | 61 (30.20) | ||
| Unknown | 668 (28.27) | 606 (28.04) | 62 (30.69) | ||
| HR status | 27.14 | <0.001* | |||
| Positive | 1,028 (43.50) | 973 (45.03) | 55 (27.23) | ||
| Negative | 154 (6.52) | 143 (6.62) | 11 (5.45) | ||
| Borderline/unknown | 1,181 (49.98) | 1,045 (48.36) | 136 (67.33) | ||
| Final status | 16.33 | <0.001* | |||
| Alive | 1,855 (78.50) | 1,677 (77.60) | 178 (88.12) | ||
| Breast cancer-related death | 145 (6.14) | 144 (6.66) | 1 (0.50) | ||
| Death | 363 (15.36) | 340 (15.73) | 23 (11.39) | ||
| iBCP | 17.41 | <0.001* | |||
| No | 2,059 (87.13) | 1,864 (86.26) | 195 (96.53) | ||
| Yes | 304 (12.87) | 297 (13.74) | 7 (3.47) | ||
Data are presented as n (%). *, P<0.05. HR, hormone receptor; iBCP, invasive breast cancer progression; PSM, propensity score matching.
OS data
In the unadjusted cohort, OS at 10 years was 74.8% (95% CI: 72.7–77.1%) (Figure 2A); the 5-year BCSS was 96.2% (95% CI: 95.3–97.1%) (Figure 2B), and iBCP rate at 10 years was 16.3% (95% CI: 14.2–18.4%) (Figure 2C). After PSM adjustment, OS at 10 years was 85.8% (95% CI: 82.2–89.6%) (Figure 2D), the 5-year BCSS was 91.6% (95% CI: 90.1–93.1%) (Figure 2E), and iBCP rate at 10 years was 9.5% (95% CI: 6.6–11.3%) (Figure 2F).

"V体育官网" Survival analysis of the RT group vs. the non-RT group
In the unadjusted cohort, OS [hazard ratio (HR): 0.285, 95% CI: 0.1789–0.430, P<0.001] (Figure 3A) and BCSS (HR: 0.040, 95% CI: 0.006–0.286, P<0.001) (Figure 3B) of the RT group was significantly higher than that of the non-RT group, while the iBCP rate in the RT group was significantly lower than that of the non-RT group (P<0.001) (Figure 3C).

As mentioned earlier, patients who received RT were often younger and more likely to have characteristics such as earlier diagnosis time, high histological grade, HR(−), etc. (Table 1). In the original data, the standardized mean value (SMD) distribution of baseline characteristics relative to treatment methods ranged from 0.10 to 0.76. To control for confounding factors other than treatment methods, PSM was applied to the data. After PSM, there were 194 patients in each of the RT and non-RT groups. The prognostic factors for the baseline characteristics were relatively balanced between the treatment groups, and the chi-square test showed no statistically significant difference (P>0.05) in each baseline characteristic between the two groups (Table S1), with the SMD distribution ranging from 0.07 to 0.19 (Figure S1).
After PSM adjustment, the OS of the RT group was significantly higher than that of the non-RT group (HR: 0.436, 95% CI: 0.264–0.718, P=0.001) (Figure 3D), as was the BCSS (HR: 0.046, 95% CI: 0.006–0.346, P<0.001) (Figure 3E). Also, the iBCP rate of the RT group was significantly lower than that of the non-RT group (P<0.001) (Figure 3F), as the 10-year iBCP rate was 4.23% (95% CI: 1.47–6.92%) in the RT group, and was 13.35% (95% CI: 6.86–19.39%) in the non-RT group.
"V体育2025版" Competitive risk model
Due to the presence of multiple outcome endpoints in this study (death, ipsilateral invasive progression, and non-invasive survival), traditional competing risk models, such as the cumulative incidence function (CIF) model, were employed to accurately estimate incidence rates. In the non-surgically treated group (Figure 4A), the 10-year risk of progression was 8.94%, and the risk of death was 12.08%. In the RT-only group (Figure 4B), the 10-year risk of progression was 0.50%, and the risk of death was 4.95%. In the group that received no local treatment (Figure 4C), the 10-year progression risk was 9.78%, and the risk of death was 12.70%. These findings indicate that in the absence of surgery, RT alone contributes to an improved prognosis by increasing BCSS and reducing the iBCP.

Subgroup analysis
As mentioned earlier, in the overall population, RT alone, relative to no local treatment, improved prognosis, including reducing iBCP and improving BCSS. To more precisely target groups with higher survival benefits for treatment, this study conducted survival benefit subgroup analysis on baseline characteristics in pre-adjustment data (Figure 5). In the overall population, RT alone was beneficial (HR =0.285, 95% CI: 0.189–0.430, P<0.001). Among them, diagnosis time between 2000–2009 (HR =0.279, 95% CI: 0.181–0.430, P<0.001), age 56–74 years (HR =0.442, 95% CI: 0.238–0.820, P=0.01), Caucasian race (HR =0.286, 95% CI: 0.186–0.441, P<0.001), upper outer quadrant (HR =0.297, 95% CI: 0.130–0.680, P=0.004), certain histological types and HR(+) (HR =0.180, 95% CI: 0.066–0.492, P<0.001) patients could also bring survival benefits through RT alone.
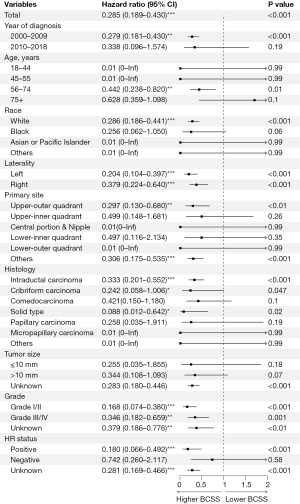
Cox regression
A multivariate Cox regression model was constructed to analyze factors such as age, diagnosis time, lesion location, race, histological type, tumor size, grade, HR status, and treatment (Figure S2). Age, race, and lesion location significantly impacted iBCP risk (P<0.05), while histological type, tumor size, grade, and treatment method were highly significant (P<0.001). Specifically, age >75 years (HR =1.560, 95% CI: 1.035–2.353, P<0.05), tumor size >10 mm (HR =2.666, 95% CI: 1.625–4.374, P<0.001), grade III/IV (HR =1.941, 95% CI: 1.462–2.577, P<0.001), and HR(−) (HR =1.616, 95% CI: 1.058–2.437, P<0.05) increased iBCP risk. Conversely, cribriform carcinoma (HR =0.418, 95% CI: 0.252–0.691, P<0.05) and RT (HR =0.134, 95% CI: 0.063–0.285, P<0.001) reduced risk. Competitive risk analysis showed groups with tumor size ≤10 mm, lower grade, and HR(+) had better prognoses, with lower iBCP rates and improved survival (Figure 6).

Discussion
This retrospective cohort analysis, based on the SEER data, suggested that the 10-year risk of progression for DCIS patients with exemption from all local therapies (including surgery and RT) was 9.78% and the risk of death was 12.70% using a competing risk model before PSM, and the 10-year iBCP was 13.35% (95% CI: 6.86–19.39%) after PSM. A recent study suggested that by integrating the Van Nuys Prognostic Index (VNPI) with National Comprehensive Cancer Network (NCCN) treatment guidelines, the NCCN’s recommendations could potentially reduce local recurrence rates by 5.6% (24). Whereas it is currently recognized that a 10-year probability of progression to invasive cancer of ≤10% is in the acceptable range (25,26). Therefore, it is not feasible to exempt all DCIS patients from local therapy.
A study by Ryser et al. based on the SEER database suggested that the 10-year ipsilateral invasive breast cancer (iIBC) rate was 12.2% (95% CI: 8.6–17.1%) in the primary analysis and 14.9% (95% CI: 10.4–19.2%) after sensitivity analyses in patients with DCIS who did not undergo topical therapy (21). The 10-year iIBC rate was closer to it among DCIS patients without local treatment in this study, including 18.12% (95% CI: 15.72–20.45%) before PSM and 13.35% (95% CI: 6.86–19.39%) after PSM.
In the unadjusted cohort, 10-year OS was significantly higher in the RT group (93.17%) compared to the non-RT group (78.09%, P=0.001). Similarly, 10-year BCSS was 99.45% in the RT group versus 90.50% in the non-RT group (P<0.001), and the 10-year iBCP rate was significantly lower in the RT group (4.23%) compared to the non-RT group (13.35%, P<0.001). These results suggest that local RT alone significantly improves prognosis in patients with DCIS who forgo surgery, which was much lower than the currently recognized range of ≤10% probability of progression to invasive cancer at 10 years as mentioned above (25,26).
Patients in the RT group tended to be younger and more likely to have high-grade tumors and a HR(−) status. To adjust for these confounding factors, PSM was applied. After PSM, there were no significant differences in baseline characteristics between the two groups (P>0.05), with standardized mean differences (SMD) ranging from 0.07 to 0.19.
Following PSM, survival analysis confirmed that 10-year OS (93.17% RT vs. 78.09% non-RT, P=0.001) and BCSS (99.45% RT vs. 90.50% non-RT, P<0.001) remained significantly higher in the RT group, while the 10-year iBCP remained significantly lower (4.23% RT vs. 13.35% non-RT, P<0.001). Notably, the 10-year iBCP in the RT group was 4.23% (95% CI: 1.47–6.92%), well below the accepted 10% probability of progression. These findings indicate that local RT alone is a viable option for DCIS patients omitting surgery.
Subgroup analysis revealed that patients aged 56–74 years (P=0.01), with tumors in the outer upper quadrant (P=0.004), or with specific histological types had a poorer prognosis when exempted from both surgery and RT. Notably, HR status interacted with the survival benefit of RT, with HR(+) patients deriving greater benefit from local RT alone.
Multifactorial Cox regression analysis identified significant factors influencing the risk of iBCP, including age over 75 years (P<0.05), tumor size >10 mm (P<0.001), histological grade III/IV (P<0.001), and HR-negative status (P<0.05), all of which increased iBCP risk. Conversely, cribriform carcinoma (P<0.05) and RT (P<0.001) significantly reduced this risk. These findings suggest the potential to stratify low-risk DCIS patients for exemption from surgery and RT while monitoring progression and survival.
Additionally, factors like race, tumor location, histological type, and HR status influenced both iBCP and survival benefits from RT, supporting individualized treatment. For instance, HR-negative patients, who have a higher iBCP risk (HR =1.616, 95% CI: 1.058–2.437, P<0.05), significantly benefit from local RT alone (HR =0.180, 95% CI: 0.066–0.492, P<0.001), highlighting its recommendation for this subgroup.
Current data indicate that the prognosis for early-stage breast cancer is very favorable. Only 1.0–2.6% of women diagnosed with DCIS will die from breast cancer within 8–10 years of diagnosis (4,5). Although up to 20–53% of patients with biopsy-diagnosed DCIS are diagnosed with invasive breast cancer within 10 years or more, there is a marked increase in the detection rate of DCIS without a corresponding decrease in the incidence of invasive breast cancer in those who are screened, implying that not all DCIS develops into invasive disease (7,8). Moreover, there is still a large heterogeneity within patients with DCIS. A recent study based on the SEER database showed that the rate of invasive progression varies widely among individual DCIS patients, with the 10-year net risk of iIBC in the absence of surgery ranging from 15% to 28%, depending on age at diagnosis and histological features (21).
Currently, there are two main types of research directed towards the treatment of breast cancer. One category is to receive BCS, followed by omission of RT. Several clinical studies have confirmed that adjuvant RT after BCS substantially reduces the absolute rate of local recurrence in the long term (2.5% vs. 4.9%; adjusted HR, 0.47, 95% CI: 0.42–0.53; P<0.001) (13,27). The other study focuses on degrading treatment, including being exempted from BCS or RT. The investigators aimed to identify a subset of patients with DCIS whose risk of aggressive progression was sufficiently low that close monitoring could reasonably be considered without the need for surgery and RT.
Currently, four clinical trials are investigating the omission of BCS and RT in low-risk DCIS patients. These trials monitor patients with low-risk clinicopathological features to assess the effects of BCS and RT exemptions on progression and survival. However, studies indicate that the 5-year iBCP rates in patients meeting the COMET, LORIS, and LORD criteria are comparable to the general population (28,29). Comparing guideline-concordant care group vs. 4.2% in the active monitoring group, the COMET trial shows that women with low-risk DCIS randomized to active monitoring did not have a higher rate of invasive cancer in the same breast at 2 years compared with those randomized to guideline-concordant care. Among DCIS patients eligible for LORIS, 20–26% had invasive cancers found in surgical specimens (30,31). Additionally, the 10-year invasive breast cancer event (IBE) rate reached 12.1% in women who met LORIS criteria and did not receive postoperative RT (32). Thus, more stringent selection criteria and precise screening for BCS exemption are necessary. Moreover, these trials did not explore RT-alone strategies for BCS-exempt patients.
In addition, some of the clinicopathological characteristics of the RT-only group in this study corresponded with the enrollment criteria of the clinical studies under investigation, including tumour size, histological grading, and HR status. Through competing risk model analysis, we found that patients with tumour size ≤10 mm, histological grading I/II, and HR(+) could still achieve a desirable iBCP with RT alone despite the relatively higher risk of progression to invasive cancer.
As mentioned previously, adjuvant RT could reduce postoperative local recurrence, including invasive breast cancer (27), but fails to improve the OS rate (31). Meanwhile, compared to the BCS alone group, only the BCS+RT group of patients with higher nuclear grade, younger age, and larger tumour volume had significantly improved OS (32). In our study, RT alone similarly reduced iBCP relative to the no local treatment group, and patients of all histological grades (Grade I/II: P<0.001; Grade III/IV: P<0.001), aged 56–74 years (P=0.01), and with HR(+) (P<0.001) benefited from RT alone. By comparison with previous studies, we hypothesize that RT may reduce the progression of DCIS to invasive carcinoma, thereby improving patient prognosis.
Several studies of post-BCS RT have shown that in the long term, adjuvant RT after BCS can substantially reduce the absolute rate of local recurrence (RT 2.5% vs. non-RT 4.9%; adjusted HR =0.47, 95% CI: 0.42–0.53; P<0.001) (7) and is effective irrespective of age at diagnosis, extent of BCS, tamoxifen use, DCIS test method, margin status, lesion, grading, acne necrosis, structure, or tumour size, adjuvant RT was effective (30). However, patients receiving adjuvant RT after BCS do not have an advantage in OS (29,30), and there is a trend towards increasing breast cancer-specific mortality in particular (22). Previous studies have suggested that local RT may limit the options for salvage after local recurrence, leading to limited subsequent palliative care, therefore resulting in a poor prognosis for patients (33,34).
However, our present study suggests that RT alone did not increase breast cancer-specific mortality but improved prognosis (HR: 0.046, 95% CI: 0.006–0.346, P<0.001). On the one hand, this may be due to the fact that RT alone limits subsequent palliative care less than adjuvant RT after BCS, reducing the adverse effect of RT on BCSS. On the other hand, in the competing risks model, the 10-year risk of death was greater than the 10-year risk of experiencing ipsilateral iBCP both in the total cohort and in each subgroup, implying that in the real-world elderly patients and patients with severe comorbidities are more inclined to adopt conservative treatment modalities, such as RT alone, which can significantly enhance their BCSS. We propose, on the basis of these studies, that some patients can obtain better treatment results by avoiding surgery and receiving RT alone. This provides a new approach to the management of some DCIS patients.
Our study has several limitations. The SEER database lacked data on HER2 status prior to 2010, surgical margin status, Ki67 expression, comorbidities, and post-iBCP treatment options, which suggests that patient selection and treatment allocation were not randomized and that the aforementioned critical biological factors were unavailable. Additionally, distinguishing primary DCIS progression from new breast cancers was difficult, possibly inflating iBCP rates. As a retrospective study, variations in biopsy techniques over time may have influenced results. Also, while our SEER-based analysis provides robust US population-level insights, its applicability to non-US healthcare systems requires cautious interpretation, considering variations in screening protocols, treatment accessibility and ethnic diversity may substantially influence outcome generalizability. Furthermore, we did not address the cost or toxic side effects of RT. Future studies could explore individualized RT regimens, such as reduced dose and duration (35), for patients with DCIS.
Conclusions
Our study found that local RT alone was effective in improving the prognosis of patients with DCIS who were exempted from surgery, and their 10-year iBCP was relatively low. Clinicopathological factors such as histological type, tumour size, histological grading, and HR status affect the survival benefit and iIBC risk of RT, thus guiding our selection of the RT patient population. Our findings may provide a more individualised treatment strategy for the treatment of DCIS, a highly heterogeneous disease, and inform future prospective clinical trials in this field.
Acknowledgments
None.
VSports在线直播 - Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-104/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-2025-104/prf
Funding: The work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-2025-104/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tomlinson-Hansen SE, Khan M, Cassaro S. Breast Ductal Carcinoma in Situ. Treasure Island (FL): StatPearls Publishing; 2025.
- American Cancer Society. Cancer Facts & Figures 2023. 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022;72:524-41. [Crossref] [PubMed]
- Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr 2010;2010:139-41. [Crossref] [PubMed]
- Worni M, Akushevich I, Greenup R, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst 2015;107:djv263. [Crossref] [PubMed]
- Lennington WJ, Jensen RA, Dalton LW, et al. Ductal carcinoma in situ of the breast. Heterogeneity of individual lesions. Cancer 1994;73:118-24. [Crossref] [PubMed]
- Narod SA, Iqbal J, Giannakeas V, et al. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol 2015;1:888-96. [V体育官网 - Crossref] [PubMed]
- Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses' Health Study. Cancer 2005;103:1778-84. [Crossref] [PubMed]
- Taghipour S, Banjevic D, Miller AB, et al. Parameter estimates for invasive breast cancer progression in the Canadian National Breast Screening Study. Br J Cancer 2013;108:542-8. [Crossref] [PubMed]
- Scribner KC, Behbod F, Porter WW. Regulation of DCIS to invasive breast cancer progression by Singleminded-2s (SIM2s). Oncogene 2013;32:2631-9. ["V体育官网入口" Crossref] [PubMed]
- Hanna WM, Parra-Herran C, Lu FI, et al. Ductal carcinoma in situ of the breast: an update for the pathologist in the era of individualized risk assessment and tailored therapies. Mod Pathol 2019;32:896-915. [Crossref] [PubMed]
- Delaloge S, Khan SA, Wesseling J, et al. Ductal carcinoma in situ of the breast: finding the balance between overtreatment and undertreatment. Lancet 2024;403:2734-46. [Crossref] [PubMed]
- Shah C, Wobb J, Manyam B, et al. Management of Ductal Carcinoma In Situ of the Breast: A Review. JAMA Oncol 2016;2:1083-8. [Crossref] [PubMed]
- Knowlton CA, Jimenez RB, Moran MS. DCIS: Risk Assessment in the Molecular Era. Semin Radiat Oncol 2022;32:189-97. [Crossref] [PubMed]
- Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol 2010;28:3762-9. [Crossref] [PubMed]
- Shah C, Bremer T, Cox C, et al. The Clinical Utility of DCISionRT(®) on Radiation Therapy Decision Making in Patients with Ductal Carcinoma In Situ Following Breast-Conserving Surgery. Ann Surg Oncol 2021;28:5974-84. [Crossref] [PubMed]
- Kanbayashi C, Thompson AM, Hwan ESS, et al. The international collaboration of active surveillance trials for low-risk DCIS (LORIS, LORD, COMET, LORETTA). J Clin Oncol 2019;37:TPS603.
- Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study. Eur J Cancer 2015;51:1497-510. [Crossref] [PubMed]
- Francis A, Thomas J, Fallowfield L, et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer 2015;51:2296-303. [VSports注册入口 - Crossref] [PubMed]
- Kanbayashi C, Iwata H. Current approach and future perspective for ductal carcinoma in situ of the breast. Jpn J Clin Oncol 2017;47:671-7. [Crossref (VSports注册入口)] [PubMed]
- Ryser MD, Weaver DL, Zhao F, et al. Cancer Outcomes in DCIS Patients Without Locoregional Treatment. J Natl Cancer Inst 2019;111:952-60. [Crossref] [PubMed]
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 2011;103:478-88. ["V体育ios版" Crossref] [PubMed]
- Maxwell AJ, Clements K, Hilton B, et al. Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol 2018;44:429-35. [Crossref] [PubMed]
- Antonini M, Barros Vasconcelos R, Mattar A, et al. Comparative Analyses of Van Nuys Prognostic Index and NCCN Guidelines in Ductal Carcinoma In Situ Treatment in a Brazilian Hospital. Life (Basel) 2025;15:432. [Crossref] [PubMed]
- Rakovitch E, Bonefas E, Nofech-Mozes S, et al. Ductal Carcinoma in Situ (DCIS)—Precision Medicine for de-Escalation. Current Breast Cancer Reports 2021;13:1-7.
- Paszat L, Sutradhar R, Zhou L, et al. Including the Ductal Carcinoma-In-Situ (DCIS) Score in the Development of a Multivariable Prediction Model for Recurrence After Excision of DCIS. Clin Breast Cancer 2019;19:35-46. ["V体育平台登录" Crossref] [PubMed]
- Sagara Y, Freedman RA, Vaz-Luis I, et al. Patient Prognostic Score and Associations With Survival Improvement Offered by Radiotherapy After Breast-Conserving Surgery for Ductal Carcinoma In Situ: A Population-Based Longitudinal Cohort Study. J Clin Oncol 2016;34:1190-6. [Crossref (V体育安卓版)] [PubMed]
- Grimm LJ, Ryser MD, Partridge AH, et al. Surgical Upstaging Rates for Vacuum Assisted Biopsy Proven DCIS: Implications for Active Surveillance Trials. Ann Surg Oncol 2017;24:3534-40. ["VSports" Crossref] [PubMed]
- Zheng L, Gökmen-Polar Y, Badve SS. Is conservative management of ductal carcinoma in situ risky? NPJ Breast Cancer 2022;8:55. [VSports - Crossref] [PubMed]
- Pilewskie M, Stempel M, Rosenfeld H, et al. Do LORIS Trial Eligibility Criteria Identify a Ductal Carcinoma In Situ Patient Population at Low Risk of Upgrade to Invasive Carcinoma? Ann Surg Oncol 2016;23:3487-93. [Crossref] [PubMed]
- Soumian S, Verghese ET, Booth M, et al. Concordance between vacuum assisted biopsy and postoperative histology: implications for the proposed Low Risk DCIS Trial (LORIS). Eur J Surg Oncol 2013;39:1337-40. [Crossref (V体育2025版)] [PubMed]
- Pilewskie M, Olcese C, Patil S, et al. Women with Low-Risk DCIS Eligible for the LORIS Trial After Complete Surgical Excision: How Low Is Their Risk After Standard Therapy? Ann Surg Oncol 2016;23:4253-61. [Crossref] [PubMed]
- Hahn E, Rodin D, Sutradhar R, et al. Can Molecular Biomarkers Help Reduce the Overtreatment of DCIS? Curr Oncol 2023;30:5795-806. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr 2010;2010:162-77. [Crossref (VSports手机版)] [PubMed]
- Li PC, Zhang Z, Cronin AM, et al. Mortality After Invasive Second Breast Cancers Following Prior Radiotherapy for DCIS. J Natl Compr Canc Netw 2019;17:1367-71. [Crossref] [PubMed]


