Two case reports of idiopathic prenatal closure of the ductus arteriosus complicated at birth by severe pulmonary hypertension successfully treated without intubation
Highlight box
Key findings
• This study presents two cases of idiopathic premature closure of the ductus arteriosus (DA) with severe pulmonary hypertension, which were successfully treated without intubation or circulatory support.
• Both neonates had persistent pulmonary hypertension at birth but responded well to noninvasive respiratory support and pharmacological treatment.
What is known and what is new?
• Premature DA closure is rare and often associated with exposure to non-steroidal anti-inflammatory drugs, while idiopathic cases are not uncommon.
• These two cases highlight the importance of early diagnosis of idiopathic DA closure, which allows for effective individualized management and avoids intubation.
What is the implication, and what should change now?
• Idiopathic premature closure of the DA should be considered in cases of unexplained persistent pulmonary hypertension of newborns with right ventricular dysfunction.
• Future research should focus on developing standardized prenatal and postnatal protocols for the management of this rare condition.
"V体育官网入口" Introduction
The ductus arteriosus (DA) is a vital fetal structure that allows blood to bypass the fluid-filled lungs VSports手机版. During fetal life, pulmonary vascular resistance (PVR) exceeds systemic resistance, directing 80–85% of right ventricular (RV) output to the systemic circulation via the DA (1) . DA patency is maintained by several mechanisms, including high levels of prostaglandins [prostaglandin E2 (PGE2), and prostacyclin 2 (PGI2)], low fetal oxygen pressure, and endothelial nitric oxide (NO) production (2-4). Disruption of these protective factors can lead to prenatal closure of the DA.
True premature closure of the DA is a rare and under-reported condition, while the estimated prevalence of prenatal duct constriction is approximately 1. 3% to 2 V体育安卓版. 5% (5,6). The use of non-steroidal anti-inflammatory drugs (NSAIDs) has been associated with premature closure or constriction of the DA, in animal and human studies (7,8). Other factors, including food rich in polyphenols and pesticides, have also been implicated.
Prenatal closure of the DA can lead to significant hemodynamic changes, including persistent pulmonary hypertension of the newborn (PPHN), RV failure, fetal hydrops and intrauterine or postnatal death (5,8,9). Despite the severity of these consequences, there are no widely accepted treatment guidelines due to the rarity of this condition. In this context, this study presents two cases of idiopathic premature closure of the DA, complicated at birth by PPHN, which were successfully treated without intubation V体育ios版. We present this article in accordance with the CARE reporting checklist (available at https://tp. amegroups. com/article/view/10. 21037/tp-2025-264/rc).
Cases presentation
Case 1
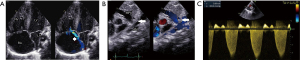
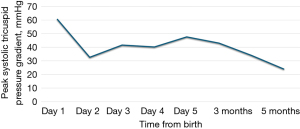
The first case concerns a female neonate born at term (39+5/7 weeks of gestation) in a context of oligohydramnios and cardiac rhythm abnormalities leading to immediate delivery. The mother, aged 33 years, had not been exposed to NSAIDs or to other potential risk factors throughout her pregnancy. Fetal ultrasounds performed at 13- and 20-weeks of gestation were normal. The immediate clinical picture at birth included respiratory distress and hypoxemia. Emergency echocardiography (delivery room) revealed moderate RV dilatation with signs of severe suprasystemic PPHN and RV dysfunction (Figure 1). In addition, the tricuspid valve and pulmonary valve appeared dysplastic, with mild to moderate regurgitation. The foramen ovale was widely patent with an exclusive right-to-left shunt, while the DA was completely closed. Chest X-ray showed mild cardiomegaly and right pneumothorax without mediastinal shift. The baby started receiving inhaled nitric oxide (iNO) at 20 ppm using only a high-flow nasal cannula (FiO2 0. 8). A continuous intravenous infusion of milrinone was also started at 0. 5 mcg/kg/min. The outcome was favorable with weaning from iNO on the second day and discontinuation of milrinone on the fourth day. The child was started on oral sildenafil (2 mg/kg/day) and follow-up echocardiograms showed a gradual decrease in pulmonary pressure with normalization of RV function. The patient was discharged from the hospital on day 9, while still on sildenafil. She was successfully weaned off the medication five months later, and repeated echocardiograms showed normal pulmonary pressure (Figure 2) VSports最新版本. At 1 year of age, she remained asymptomatic, with good neurological development and normal RV systolic pressure of 24 mmHg.
Case 2
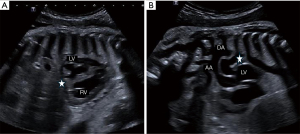
The second case involved a full-term male neonate. Routine prenatal screening, including fetal cardiac ultrasound at 12 and 22 weeks, was normal. The mother, aged 28 years, has not been exposed to NSAIDs or to other potential risk factors during pregnancy and has no significant medical history V体育平台登录. At 40 weeks of gestation +1/7, fetal echocardiography was performed due to cardiac rhythm abnormalities. Spontaneous premature closure of the DA was strongly suspected as it was impossible to demonstrate colored flow indicating patency of the DA (Figure 3). Other abnormalities were observed, including mild pericardial effusion, marked RV hypertrophy and dilatation, and moderate RV systolic dysfunction. The tricuspid valve was also dysplastic and showed mild regurgitation.
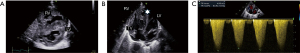
In this setting of suggestive hemodynamic compromise, an emergency cesarean section was indicated, at birth, the newborn immediately presented with respiratory distress without abnormalities on chest X-ray, while transthoracic echocardiography performed within 10 minutes of life confirmed closure of the DA. There was an exclusively right-to-left shunt through the foramen ovale. The RV was severely hypertrophic, slightly dilated and dysfunctional. The tricuspid valve was confirmed as dysplastic with moderate regurgitation and signs of suprasystemic pulmonary hypertension (Figure 4).
A trial of prostaglandin E1 at 0.02 mcg/kg/min for several hours failed to reopen the DA. However, the infant’s condition improved rapidly with non-invasive continuous positive airway pressure (CPAP) ventilation alone, later transitioning to low-flow nasal cannula. As a result, iNO and milrinone were not administered, and follow-up echocardiograms showed a gradual decrease in pulmonary systolic pressure, with normalization of RV systolic function. The baby was finally discharged on the 9th day without any medication, with normal pulmonary pressure one month later. A year later, he remained asymptomatic with normal development and an echocardiogram.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from parents of the two patients for the use of anonymized clinical data and the publication of this case report and accompanying images, in accordance with the institutional procedure of the Geneva University Hospitals. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Idiopathic premature closure of the DA can manifest at birth as PPHN caused by failure or delay in the normal transition from fetal to neonatal circulation. PPHN is characterized by persistently elevated PVR and a right-to-left shunt resulting in systemic hypoxemia. Although this condition can be life-threatening or require extracorporeal membrane oxygenation (ECMO) (10), we report two cases of premature closure of the DA that appear remarkable for two reasons: (I) they were not associated with maternal drug exposure; (II) their postnatal clinical course was favorable due to medical care that avoided intubation, which contrasts with various previous reports.
Although the clinical findings in our cases could mimic those of a twin-to-twin transfusion syndrome (TTTS), particularly in the recipient twin due to volume overload and right heart strain, this diagnosis was excluded because both neonates were singletons. Moreover, laboratory values did not support TTTS-like hemodynamic alterations. Hemoglobin values were within normal limits for both patients [case 1: 141 g/L, hematocrit (Hct): 43.3%; case 2: 165 g/L, Hct 50.5%], ruling out significant hemoconcentration or volume imbalance, and further supporting the idiopathic nature of the ductal closure in both cases.
Premature closure of the DA disrupts fetal circulation and has repercussions on neonatal outcomes, although its incidence remains unknown, with clinical data mainly derived from case reports and case series (2,3,5,9,11). It induces reactive pulmonary vascular remodeling, as increased pulmonary blood flow in utero interacts with a normally constricted pulmonary vascular system. The resulting increase in pulmonary artery pressure and flow pulsatility contributes to arterial muscularization, adventitial thickening, and pulmonary arteriolar remodeling, as well as potentially severe RV afterload. This increased afterload can contribute to pulmonary valve leakage, with remodeling and dysplasia of the valve leaflets. At birth, the pulmonary vasculature remains abnormally narrowed, impeding the expected postnatal drop in PVR following lung expansion and oxygenation. The RV continues to face increased afterload contributing to RV ischemia and dysfunction, papillary muscle dysfunction and tricuspid regurgitation, as observed in our two patients (12,13). In addition, recent clinical studies suggest that the severity of PPHN may be influenced by the timing and degree of ductal constriction or closure, even when not hemodynamically significant (13).
Premature DA occlusion was diagnosed before birth in only one of our two cases, using the standard sagittal view of the ductal arch view, which normally includes the DA in continuity with the main pulmonary trunk and descending aorta. In this case, color and pulsed Doppler echocardiography revealed absence of flow through the DA. Additional echocardiography findings included RV hypertrophy and dilatation, as well as dysplasia and varying degrees of regurgitation of both tricuspid and pulmonary valves.
The timing of diagnosis varies considerably during fetal life or may not be made at all, although ductal constriction causing hydrops may also be detected on routine examinations (14). Prolonged fetal DA constriction has been associated with increased perinatal morbidity and mortality ranging from 10% to 25% (9). On the other hand, idiopathic premature closure of the DA occurring before 37 weeks of gestation may be followed, in some cases, by reopening of the DA, which would suggest, at least initially, simple and careful monitoring of the fetus’s well-being (15). In the absence of prenatal diagnosis, premature DA closure can only be suspected at birth in the presence of signs of PPHN, including RV hypertrophy and systolic dysfunction, right-to-left atrial shunt through a patent foramen ovale, and lack of DA patency on early echocardiography, as observed in our second case.
The main intervention in the event of a poorly tolerated premature fetal closure of the DA is early induction of labor to mitigate cardiac and pulmonary damage and improve outcomes. The latter depends largely on the timing of delivery and the severity of PPHN as previously emphasized. After birth, management depends on the severity of the PPHN and cardiac dysfunction, and aims to reduce PVR and RV afterload, minimize extrapulmonary and intrapulmonary shunts, and optimize cardiac output and organ perfusion (16).
Based on clinical manifestations and echocardiographic findings, these two cases of premature closure of the DA highlight the possibility of implementing effective individualized treatment plans that avoid intubation or ECMO support. In case 1, the neonate was treated with iNO, high-flow nasal cannula (FiO2 0.80), and milrinone to optimize RV function. Oral sildenafil was then introduced due to persistent moderate pulmonary hypertension, which gradually resolved over five months. In case 2, despite an initial fetal echocardiographic indicating severe RV dysfunction, the neonate showed rapid improvement with non-invasive ventilation, but with high FiO2 (0.8), rendering administration of iNO or milrinone unnecessary. These favorable long-term outcomes are consistent with recent findings (15) that early diagnosis and individualized management of DA closure may reduce the risk of subsequent complications. Both newborns showed normal echocardiographic findings and neurological development at one-year follow-up.
Conclusions
Prenatal closure of the DA in the absence of known triggering factors presents a broad clinical spectrum. Clinicians should suspect this condition in the presence of RV hypertrophy, dysplastic tricuspid valve with regurgitation on prenatal echocardiography, or postnatal hypoxemia with PPHN in the absence of underlying pulmonary or cardiac diseases. Rapid delivery may be crucial, while early and appropriate medical management, including noninvasive ventilation, administration of iNO and milrinone, may improve neonatal outcomes. Future research focused on standardizing diagnostic and treatment protocols is recommended to improve neonatal outcomes of this rare entity.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-264/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-264/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-264/coif). Maurice Beghetti has received speaker fees and/or honoraria from Actelion Pharmaceuticals Ltd (Johnson & Johnson), AOP Orphan Pharmaceuticals GmbH, Merck (MSD), Gossamer Bio, GlaxoSmithKline (GSK), and Liquidia. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from parents of the two patients for the use of anonymized clinical data and the publication of this case report and accompanying images, in accordance with the institutional procedure of the Geneva University Hospitals. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Battistoni G, Montironi R, Di Giuseppe J, et al. Foetal ductus arteriosus constriction unrelated to non-steroidal anti-Inflammatory drugs: a case report and literature review. Ann Med 2021;53:860-73. [Crossref] [PubMed]
- Bakas AM, Healy HM, Bell KA, et al. Prenatal duct closure leading to severe pulmonary hypertension in a preterm neonate-a case report. Cardiovasc Diagn Ther 2020;10:1691-5. [Crossref] [PubMed]
- Le Duc K, Gilliot S, Baudelet JB, et al. Case Report: Persistent Pulmonary Hypertension of the Newborn and Narrowing of the Ductus Arteriosus After Topical Use of Non-Steroidal Anti-Inflammatory During Pregnancy. Front Pharmacol 2021;12:756056. [Crossref] [PubMed]
- Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation 2006;114:1873-82. [Crossref] [PubMed]
- Gewillig M, Brown SC, De Catte L, et al. Premature foetal closure of the arterial duct: clinical presentations and outcome. Eur Heart J 2009;30:1530-6. [Crossref] [PubMed]
- Zielinsky P, Sulis NM, Martins CM, et al. Fetal ductal constriction in the third trimester of pregnancy: a prevalence study. J Perinatol 2024;44:444-5. [Crossref] [PubMed]
- Wen J, Guo X, Cai S, et al. Fetal Ductus Arteriosus Premature Constriction. Int Heart J 2022;63:722-8. [Crossref] [PubMed]
- Lopes LM, Carrilho MC, Francisco RP, et al. Fetal ductus arteriosus constriction and closure: analysis of the causes and perinatal outcome related to 45 consecutive cases. J Matern Fetal Neonatal Med 2016;29:638-45. [Crossref] [PubMed]
- Ishida H, Kawazu Y, Kayatani F, et al. Prognostic factors of premature closure of the ductus arteriosus in utero: a systematic literature review. Cardiol Young 2017;27:634-8. [Crossref] [PubMed]
- San Geroteo J, Rambaud J. Premature Closure of the Ductus Arteriosus and Veno-Arterial Extracorporeal Membrane Oxygenation in Critically Ill Neonates: A 10-Year Single-Center Retrospective Study. Pediatr Cardiol 2024; [Crossref]
- Gewillig M, Brown SC, Roggen M, et al. Dysfunction of the foetal arterial duct results in a wide spectrum of cardiovascular pathology. Acta Cardiol 2017;72:625-35. [Crossref] [PubMed]
- Aoki H, Kawataki M, Kim K, et al. Reopening of ductus arteriosus in idiopathic premature constriction or closure of ductus arteriosus: A case series. J Neonatal Perinatal Med 2023;16:75-80. [Crossref] [PubMed]
- Chesi E, Rossi K, Ancora G, et al. Patent ductus arteriosus (also non-hemodynamically significant) correlates with poor outcomes in very low birth weight infants. A multicenter cohort study. PLoS One 2024;19:e0306769. [Crossref] [PubMed]
- Kadiyani L, Rana A, Dadhwal V, et al. Fetal ductal constriction: An ominous but treatable entity! Ann Pediatr Cardiol 2024;17:227-8. [Crossref] [PubMed]
- Kikuchi N, Goto T, Katsumata N, et al. Correlation between the Closure Time of Patent Ductus Arteriosus in Preterm Infants and Long-Term Neurodevelopmental Outcome. J Cardiovasc Dev Dis 2024;11:26. ["VSports app下载" Crossref] [PubMed]
- Singh Y, Lakshminrusimha S. Pathophysiology and Management of Persistent Pulmonary Hypertension of the Newborn. Clin Perinatol 2021;48:595-618. [Crossref (VSports手机版)] [PubMed]


