Enhancing early mother’s own milk (MOM) use in NICU preterm infants with colostrum oral immune therapy (COIT): evidence summary
"VSports注册入口" Highlight box
Key findings
• The evidence of enhancing early mother’s own milk (MOM) use in neonatal intensive care unit (NICU) preterm infants with colostrum oral immune therapy (COIT) was summarized.
What is known and what is new?
• Early application of MOM during the postpartum period offers multiple benefits for preterm infants in NICU, and COIT is the main method to achieve this goal. However, there is currently no consolidated evidence to guide the implementation of such bundled practices VSports在线直播.
• We summarized the existing 68 pieces of relevant evidence from eight aspects, including personnel training and health education, informed consent, MOM collection and lactation support, requirements for MOM storage in the obstetric ward and at home, MOM transport, MOM reception, MOM handling within the NICU, and the implementation of COIT.
What is the implication, and what should change now?
• We have established a bundled evidence base to support the early application of MOM in the NICU. Current management practices for MOM predominantly reference human milk bank management literature from various countries, but lack high-quality primary studies and systematic reviews. During the evidence-based clinical translation process, contextual adaptation and tailored application of evidence are essential. Future research should prioritize conducting primary studies to address low-quality evidence gaps and systematically translate existing evidence into clinical practice through multidisciplinary theoretical frameworks, ultimately integrating these practices into routine neonatal care protocols VSports app下载.
Introduction
According to the World Health Organization (WHO), one in ten newborns has been born preterm in the past decade (1), with the majority requiring necessary care in neonatal intensive care unit (NICU) (2). Fortunately, advancements in neonatal rescue techniques have significantly improved survival rates for preterm infants—even extremely preterm ones (3). However, this progress has been accompanied by an increased incidence of life-threatening complications arising from prematurity-related immaturity, such as retinopathy of prematurity (ROP), late-onset sepsis (LOS), bronchopulmonary dysplasia (BPD), and necrotizing enterocolitis (NEC), etc V体育官网. (4).
It is critical to initiate early postnatal breastfeeding for preterm infants, as it serves as a vital strategy to reduce neonatal morbidity and mortality while enhancing long-term survival (5). Among these, mother’s own milk (MOM) is not only the best nutrition for NICU preterm infants, but also a personalized medicine for preventing and treating prematurity-related diseases (6). However, there are multiple factors that contribute to the delay of MOM feeding. Physiologically, preterm infants often exhibit suck-swallow-breathe incoordination as well as immature neurological and digestive systems, necessitating parenteral nutrition and/or nasogastric tube feeding during early life (2). Inability to direct breastfeeding significantly increases the reliance on artificial pumping methods VSports手机版. Simultaneously, mothers face challenges in maintaining and establishing breastfeeding due to perinatal complications, prolonged mother-infant separation, psychological stress, and insufficient breastfeeding education (7,8). Consequently, promoting early lactogenesis in mothers thereby ensuring the timely and safe application of MOM is a critical component of NICU-based MOM feeding protocols. The achievement of this objective has been proven to be more complex than anticipated, as it entails complex procedures including health education, lactation promotion, and standardized protocols for breast milk management (9), requiring close collaboration among multiple stakeholders.
For preterm infants who cannot be fed orally, colostrum oral immune therapy (COIT) represents an effective way to deliver the benefits of MOM. COIT is a safe and simple treatment, first proposed by Rodriguez et al. (10), which involves administering small amounts of colostrum into the newborn’s mouth by syringes or sterile swabs in the first few days after birth V体育安卓版. Upon interaction with the oral mucosa, immunomodulatory components in colostrum engage with oropharyngeal lymphoid tissues, thereby modulating neonatal immune responses and inflammatory pathways (4).
Several studies exploring the development of NICU-based protocols for early MOM utilization have primarily adopted Quality Improvement (QI) approaches (11-13). However, the clinical applicability of such research is often confined to the research institution itself, with limited feasibility for translation to other nursing settings. Therefore, this study aims to systematically synthesize evidence on improving the early use of MOM in NICU preterm infants with COIT by evidence-based nursing methodology, ultimately providing a general reference for the development and standardization of early MOM use in NICU preterm infants. We present this article in accordance with the PRISMA reporting checklist (available at https://tp. amegroups. com/article/view/10. 21037/tp-2025-245/rc) V体育ios版.
VSports手机版 - Methods
Problem establishment
The PIPOST model (14) was employed to construct evidence-based issues. The specific components are as follows. Population (P): NICU hospitalized preterm infants. Intervention (I): interventions aimed at enhancing the early use of MOM in NICU preterm infants. Professionals (P): clinical healthcare providers and families of preterm infants. Outcomes (O): management practices for MOM, stakeholder application of evidence, incidence of clinical outcomes in NICU preterm infants. Setting (S): maternity wards, NICU wards and families. Type of evidence (T): clinical decisions, guidelines, systematic reviews, expert consensuses, evidence summaries, recommended practices. This study has been registered with the Center for Evidence-Based Nursing at Fudan University for evidence summary (ES20244612).
Literature retrieval
Computerized hierarchical retrieval was conducted based on the “6S” search pyramid (15). Search databases included UpToDate, Scottish Intercollegiate Guidelines Network (SIGN), Guidelines International Network (GIN), National Institute for Health and Care Excellence (NICE), National Association of Neonatal Nurses (NANN), Academy of Breastfeeding Medicine (ABM), Registered Nurses Association of Ontario (RNAO), Medlive, Cochrane Library, Joanna Briggs Institute (JBI) Evidence-Based Practice (EBP) Database (via Ovid), PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfangdata, China Biology Medicine disc (CBM). The search terms were determined according to the topics. English search terms included “preterm infant/low birth weight” “mother’s own milk/breast milk collect*/lactat* promot*/breast milk manag*” ‘colostrum oral immun*/colostrum oral care/colostrum oropharyngeal immun*’. Chinese databases utilize corresponding Chinese search terms for retrieval. The retrieval strategy combines both subject terms and free text terms, with appropriate adjustments made to the search vocabulary according to database requirements. Taking PubMed as an example, the retrieval strategy is illustrated in Figure 1. The search timeframe was set from January 1, 2014 to August 31, 2024, with the final retrieval conducted on September 13, 2024.
"VSports在线直播" Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) language must be Chinese or English; (II) types of literature should include clinical decisions, guidelines, systematic reviews, expert consensuses, evidence summaries; (III) time limit was January 1, 2014 to August 31, 2024.
The exclusion criteria were as follows: (I) incomplete information or unobtainable full-text; (II) the literature has been updated, repetitively published or interpreted as guidelines; (III) the literature failed to pass quality evaluation.
Literature screening
Two researchers independently screened the literature according to the literature inclusion criteria and cross-checked their selections. In case of disagreement, a third researcher was consulted for resolution. The specific process included the following steps: (I) all records retrieved from the literature search were imported into NoteExpress, where duplicates were automatically and manually removed; (II) the de-duplicated records were imported into the Rayyan tool (16), and screening was performed based on titles and abstracts; (III) full-text articles were reviewed to determine the eligible literature.
Literature quality evaluation
Two researchers independently evaluated the literature for quality assessment. In case of disagreement, a discussion with a third researcher was conducted to reach a decision. The evaluation was conducted using the following tools: (I) the Appraisal of Guidelines for Research & Evaluation II (AGREE II) (17) was used for the quality evaluation of guidelines; (II) the quality evaluation of expert consensuses was performed using the JBI Evidence-Based Health Care Center Expert Consensus Evaluation Criteria (18); (III) the Critical Appraisal for Summaries of Evidence (CASE) (19) was used for the quality assessment of clinical decisions and evidence summaries; (IV) the quality of systematic reviews was assessed using the JBI Evidence-Based Health Care Center Systematic Review Authenticity Assessment Tool (18). Low-quality literature was further excluded based on the evaluation results.
Two researchers independently extracted and integrated the evidence, following the principles outlined below (20): (I) when evidence conclusions were consistent, they were summarized in a simple and easily understandable manner; (II) when evidence conclusions were complementary, they were integrated according to the logical relationship; (III) when evidence conclusions conflict, priority is given to the best available, high-quality, and most recent authoritative evidence.
The evidence grading and recommendation strength were determined using the JBI Evidence Pre-Grading and Recommendation level System (2014 version) (21). This tool categorizes the evidence levels from Level 1 to 5 based on the type of literature, and classifies the recommendation strength into Level A (strong recommendation) and Level B (weak recommendation) according to the FAME structure (Feasibility, Appropriateness, Meaningfulness, and Effectiveness).
- Classification of evidence levels (22):
- Level 1: experimental designs:
- 1a: systematic review of randomized controlled trials (RCTs).
- 1b: systematic review of RCTs and other study designs.
- 1c: RCT.
- 1d: pseudo-RCTs.
- Level 2: quasi-experimental designs:
- 2a: systematic review of quasi-experimental studies.
- 2b: systematic review of quasi-experimental and other lower study designs.
- 2c: quasi-experimental prospectively controlled study.
- 2d: pre-test-post-test or historic/retrospective control group study.
- Level 3: observational-analytic designs:
- 3a: systematic review of comparable cohort studies.
- 3b: systematic review of comparable cohort and other lower study designs.
- 3c: cohort study with control group.
- 3d: case-controlled study.
- 3e: observational study without a control group.
- Level 4: observational-descriptive studies:
- 4a: systematic review of descriptive studies.
- 4b: cross-sectional study.
- 4c: case series.
- 4d: case study.
- Level 5: expert opinion and bench research:
- 5a: systematic review of expert opinion.
- 5b: expert consensus.
- 5c: bench research/single expert opinion.
- Level 1: experimental designs:
- Recommendation ratings (23):
- Grade A: a ’strong’ recommendation for a certain health management strategy.
- Grade B: a ‘weak’ recommendation for a certain health management strategy.
Results
VSports手机版 - General characteristics of the included literatures
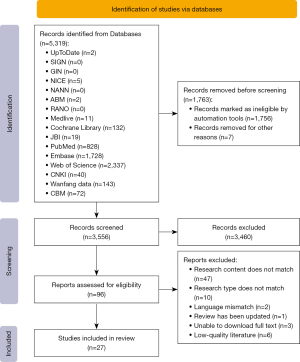
The initial search retrieved 5,319 literature records, which were progressively screened through deduplication, title/abstract review, full-text evaluation, and quality assessment to ultimately include 27 eligible studies. The literature screening process and results are presented in Figure 2. Among the included literature, there were two clinical decisions, one guideline, nineteen systematic reviews, three evidence summaries, and two expert consensuses. The general characteristics of the included literatures are shown in Table 1.
Table 1
| Included literature | Year | Topics | Source | Type of literature |
|---|---|---|---|---|
| A Abrams S (24) | 2024 | Breast milk expression for the preterm infants | UpToDate | Clinical decision |
| A Abrams S (25) | 2024 | Breastfeeding the preterm infants | UpToDate | Clinical decision |
| Yang PY et al. (26) | 2018 | Hospitalized newborns’ breastfeeding | CNKI | Clinical guideline |
| Anne RP et al. (27) | 2024 | Effect of COIT on sepsis in preterm infants | PubMed | Meta-analysis |
| Gomez JA et al. (28) | 2024 | Effect of fresh MOM versus frozen/pasteurized MOM on preterm infants | PubMed | Systematic review |
| Fu ZY et al. (4) | 2023 | Impact of COIT on clinical outcomes in preterm infants | PubMed | Meta-analysis |
| Lang Y et al. (29) | 2023 | Effect of COIT on LOS in preterm infants | CNKI | Meta-analysis |
| Kumar J et al. (30) | 2023 | Impact of COIT on clinical outcomes in preterm infants | Web of Science | Meta-analysis |
| Cai M et al. (31) | 2022 | Effect of breast milk oral care on mechanically ventilated preterm infants | Embase | Meta-analysis |
| Huo M et al. (32) | 2022 | Efficacy and safety of COIT in preterm infants | Embase | Meta-analysis |
| Zhang XY et al. (33) | 2022 | Effect of COIT on gastrointestinal feeding outcomes in preterm infants | Wanfang data | Meta-analysis |
| Zhou WJ et al. (34) | 2022 | Methods and effects of breast milk disinfection for cytomegalovirus infection | CNKI | Meta-analysis |
| Xavier Ramos MS et al. (35) | 2021 | Effect of COIT on reducing the time to achieve full enteral nutrition in ELBW infants | Web of Science | Meta-analysis |
| Cai HW et al. (36) | 2021 | Effect of oral immunotherapy on ventilator-associated pneumonia in preterm infants | Wanfang data | Meta-analysis |
| Tao J et al. (37) | 2020 | Effect of COIT on necrotizing enterocolitis, late-onset sepsis, and mortality in preterm infants | PubMed | Meta-analysis |
| Panchal H et al. (38) | 2019 | Impact of COIT on clinical outcomes in preterm infants | Web of Science | Meta-analysis |
| Li YY et al. (39) | 2019 | Effect of COIT in preterm infants | Wanfang data | Meta-analysis |
| Wang Q et al. (40) | 2019 | Effect of COIT in preventing NEC in preterm infants | CBM | Meta-analysis |
| Garg BD et al. (41) | 2018 | Effect of COIT on NEC in ELBW infants | PubMed | Meta-analysis |
| Nasuf AWA et al. (42) | 2018 | Effect of COIT on the mortality and morbidity of preterm infants | Cochrane Library | Systematic review |
| Xu SH et al. (43) | 2018 | Effect of oral immunotherapy on preterm infants | CBM | Meta-analysis |
| Becker GE et al. (44) | 2016 | Methods for milk expression in lactating women | Cochrane Library | Meta-analysis |
| Ji RT et al. (45) | 2024 | Evidence summary for COIT in preterm infants | CNKI | Evidence summary |
| Fu ZY et al. (46) | 2021 | Evidence summary for maintaining lactation during mother-infant separation in ELBW infants | CBM | Evidence summary |
| Jin YM et al. (47) | 2020 | Evidence summary for breast milk collection of preterm infants during mother-infant separation | CBM | Evidence summary |
| Cao Y et al. (48) | 2021 | Expert consensus on the use of breast milk in NICU | Medlive | Expert consensus |
| Perinatal Medicine Branch of Chinese Medical Association (49) | 2021 | Expert consensus on common maternal infections and breastfeeding guidance | Wanfang data | Expert consensus |
CBM, China Biology Medicine disc; CNKI, China National Knowledge Infrastructure; COIT, colostrum oral immune therapy; ELBW, extremely low birth weight; LOS, late-onset sepsis; MOM, mother’s own milk; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit.
Results of literature quality evaluation
Quality evaluation results of evidence summaries and clinical decisions
The evaluation results of the two clinical decisions and three evidence summaries are shown in Table 2.
Table 2
| Included literature | Number of items | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| A Abrams S (24) | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| A Abrams S (25) | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| Ji RT et al. (45) | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Fu ZY et al. (46) | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Jin YM et al. (47) | Yes | Yes | No | Not completely | Yes | Yes | Yes | No | Yes | Yes |
Items contents (19): 1. Is the summary specific in scope and application? 2. Is the authorship of the summary transparent? 3. Are the reviewer(s)/editor(s) of the summary transparent? 4. Are the search methods transparent and comprehensive? 5. Is the evidence grading system transparent and translatable? 6. Are the recommendations clear? 7. Are the recommendations appropriately cited? 8. Are the recommendations current? 9. Is the summary unbiased? 10. Can this summary be applied to your patient(s)?
Quality evaluation results of the guideline
The evaluation results of the guideline are shown in Table 3.
Table 3
| Included literature | Percentage of field standardization % | ≥60% field | ≥30% field | Recommendation level | |||||
|---|---|---|---|---|---|---|---|---|---|
| Scopes and objects | Participant | Rigor of the guidelines | Clarity of guidelines | Application of guidelines | Independence of the guide | ||||
| Yang PY et al. (26) | 75.9 | 38.9 | 79.9 | 79.6 | 36.1 | 41.7 | 3 | 6 | B |
Quality evaluation results of systematic reviews
The evaluation results of the 19 systematic reviews were mostly ‘Yes’, indicating a generally high quality. The main items rated ‘No’ were Item 9 (32,35,36,39,43) (Was the likelihood of publication bias assessed?) and Item 10 (32,35,38-40,43) (Were recommendations for policy and/or practice supported by the reported data?). Detailed results are available in Table S1.
Quality evaluation results of expert consensus
All items in the two expert consensus articles were rated ‘Yes’, indicating high quality.
VSports在线直播 - Summary and description of evidence
Ultimately, 68 pieces of evidence were synthesized and conducted across eight categories, including personnel training and health education, informed consent, MOM collection and lactation support, requirements for MOM storage in the obstetric ward and at home, MOM transport, MOM reception, MOM handling within the NICU, and the implementation of COIT, as shown in Table 4.
Table 4
| Category | Evidence content | Evidence level | Recommendation level |
|---|---|---|---|
| Personnel training and health education | 1. Establish a perinatal breast-feeding guidance team (46) | 5c | B |
| 2. Conduct regular training for all staff in obstetrics and neonatology to ensure they possess the necessary knowledge, skills, and competencies to support preterm infants MOM (25,46,47) | 5c | B | |
| 3. Ensure that all staff in obstetrics and neonatology clearly identify the target population for COIT, such as preterm infants who cannot be fed orally due to feeding intolerance, mechanical ventilation, or instability of the internal environment caused by low perfusion in the early postnatal period (45,48) | 5b | A | |
| 4. Integrate education on breastfeeding, especially COIT, as a routine component in prenatal care for expectant mothers at risk of preterm birth and their families (24,46-48) | 5c | A | |
| 5. Ensure that mothers and families are fully informed of the correct procedures for MOM collection, storage, and transport through health education (24,25) | 5c | A | |
| 6. Develop written educational materials for preterm infant MOM, providing comprehensive knowledge on COIT implementation and other aspects of MOM for preterm infants (24,46) | 1b | B | |
| 7. Emphasize the role of COIT in the training and education content, such as reducing the incidence of feeding intolerance, NEC, LOS, and other complications during preterm infants’ hospitalization, while also shortening the time to initiate oral feeding, achieve full oral feeding, and reduce the length of hospitalization, all without adverse reactions (4,27,30-33,35-37,39,40,43) | 1a | A | |
| Informed consent | 8. Obtain informed consent for breastfeeding from the preterm infant’s family before implementing breastfeeding (MOM) (26,46) | 5b | B |
| MOM collection and lactation support | 9. Distribute lactation logs to help preterm infant mothers self-monitor the effects of MOM collection (26,47,48) | 5b | A |
| 10. Ensure proper handwashing before MOM collection (24,26,47) | 1c | A | |
| 11. Clean and disinfect collection equipment before use, with options including disinfectant machines, microwave sterilization bags, or boiling in water for 15 to 20 minutes (24,26,47) | 5b | A | |
| 12. Assist mothers in choosing a breast pump according to their personal preferences (26,46) | 1b | A | |
| 13. Recommend using a hospital-grade electric breast pump (26,46) | 3b | A | |
| 14. Recommend using a double-sided breast pump (24,26,46) | 2b | A | |
| 15. Provide breast pumps and areas for preterm infant mothers during hospitalization (26,46) | 5b | A | |
| 16. Adjust the breast pump mode to simulate infant sucking during lactation initiation (24,26,46) | 1c | B | |
| 17. Choose the correct-size breast shield to ensure that the nipple and areola move freely in and out of the shield, without contacting the sides of the nipple (24,25) | 5c | A | |
| 18. Combine hand expression with breast pump use to adequately stimulate lactation (24–26) | 1b | A | |
| 19. Begin milk collection within six hours after delivery (24,26,46) | 2b | A | |
| 20. Perform hand expression or breast pumping at least eight times a day, every two to three hours, with at least one session during the night (24-26,46,47) | 5b | A | |
| 21. Before lactation initiation, use a double-sided breast pump for 10 to 15 minutes per session (24,47) | 1c | A | |
| 22. After lactation initiation, use a double-sided breast pump for at least 15 minutes per session until only a small amount of milk is expressed (24) | 1c | A | |
| 23. Perform breast relaxation, warm compresses, and massage before pumping to promote lactation (24-26) | 5c | A | |
| 24. Allow mothers to pump milk at their infant’s bedside (26,46,47) | 5b | A | |
| 25. Provide mothers with photos of their infants (26,46,47) | 5b | A | |
| 26. Offer peer support for preterm infant mothers (26,46,47) | 5b | A | |
| 27. Implement kangaroo care as early as possible when conditions allow for mother-infant contact (24-26) | 1b | A | |
| Requirements for MOM storage in the obstetric ward and at home | 28. Storage containers should be clean, dry, sealable, and made of materials that meet infant food-grade standards, free from bisphenol A. Glass or polypropylene containers are recommended, while polyethylene and stainless steel materials are not recommended (24,48) | 5c | A |
| 29. Select containers with appropriate capacities to store MOM based on infant’s feeding volume (26) | 5b | A | |
| 30. When using syringes for COIT, withdraw 0.2 mL per syringe, collecting six to twelve syringes per day, labeling them, and storing them at four degrees (48) | 5b | A | |
| 31. Fresh MOM should be used within four hours at room temperature (16 to 29 ℃) (24,48) | 5c | A | |
| 32. MOM stored at one to four degrees should be used within 72 to 96 hours (24,48) | 5c | A | |
| 33. Frozen MOM at –18 to –20 ℃ should be used within six to nine months (24,48) | 5c | A | |
| 34. Freeze MOM immediately if not used within 24 hours of collection (26) | 5b | A | |
| 35. Do not fill storage containers more than three fourths full to prevent container damage due to the expansion of frozen milk (26) | 5b | A | |
| 36. Store each collection of MOM separately (26) | 5b | A | |
| 37. After collecting MOM, label the storage container with the infant’s name, hospitalization number, milk volume, collection time, and the medications currently taken by the mother (24,26) | 5c | A | |
| 38. Immediately place collected MOM in the refrigerator, organizing it in chronological order of collection. Store it inside the refrigerator, not in the door (48) | 5b | A | |
| 39. Instruct parents of preterm infants not to store MOM for extended periods at home or outside the supervision of hospital staff to minimize the risk of accidental freezing, thawing, or contamination (24) | 5c | A | |
| MOM transport | 40. Select transport container based on the actual situation to keep the MOM refrigerated or frozen. Generally, use cooling bags and ice packs, and if the time exceeds 18 hours, use dry ice (26,48) | 5b | A |
| 41. When the container is larger, fill the empty spaces with a clean dry towel (26,48) | 2c | A | |
| 42. Thoroughly clean the transport container, and ensure that the transporter washes their hands thoroughly to prevent contamination (24) | 5c | A | |
| 43. Transporters should deliver the MOM to the NICU along with the mother’s relevant documents, visiting card, or milk delivery card (48) | 5b | A | |
| MOM reception | 44. Facilities with the necessary resources should establish an electronic system for NICU breastfeeding protocol (milk delivery code-milk reception code- portioning code), transitioning from manual to electronic records to ensure information accuracy and feeding safety (48) | 5b | B |
| 45. Verify the milk information against the delivery card (48) | 5b | A | |
| 46. Verify that the milk labeling is clear and complete, check the milk volume and status (refrigerated or frozen), and ensure the packaging is intact (26,48) | 5b | A | |
| 47. Record the infant’s name, amount of MOM received, label compliance, packaging condition and milk status (26) | 5b | A | |
| 48. Facilities with the necessary resources should establish a unique QR code for each bottle of milk, including the infant’s name, hospitalization number, bed number, pumping time, milk volume, and status (48) | 5b | B | |
| MOM handling within the NICU | |||
| Principles | 49. Strictly follow aseptic principles when handling MOM (26) | 3b | A |
| 50. Use MOM according to the collection time, prioritizing colostrum and fresh milk (26) | 1c | A | |
| MOM sterilization | 51. Perform either traditional pasteurization or short-time pasteurization (62.5 ℃ for five seconds) on the MOM before use for ELBW or extremely preterm infants (34,48,49) | 5b | A |
| Management of MOM storage in NICU | 52. Allow only authorized staff to enter and exit the MOM storage area to ensure milk safety (26) | 5b | A |
| 53. Equip the refrigerator with an alarm system to maintain the normal temperature. Record the temperature and clean the refrigerator periodically (26) | 5b | A | |
| 54. Store fresh MOM at room temperature between 16 to 29 ℃, with a shelf life of four hours (26) | 1b | A | |
| 55. Store MOM at four degrees with a shelf life of 48 hours (48) | 5b | A | |
| 56. Store MOM at −20 ℃ when freezing, with a shelf life of three months (26) | 3b | A | |
| 57. Place the received MOM in an orderly manner and properly position it within the refrigerator. Facilities with the necessary conditions may include the refrigerator placement information in the milk reception code (48) | 5b | A | |
| MOM thawing | 58. Thaw MOM in a refrigerator, cold water, warm water (≤37 ℃), or a bottle warmer. Ensure that the liquid level of the thawing water does not exceed the bottle opening. Do not use a microwave to thaw MOM (26) | 5b | A |
| 59. Label the thawing date and time on the MOM bottle (24) | 5c | A | |
| 60. Store thawed MOM at four degrees in the refrigerator and use it within 24 hours (26,48) | 3b | A | |
| 61. Do not refreeze thawed MOM (26) | 5b | A | |
| MOM portioning | 62. Portion breast milk according to medical instructions, labeling each syringe/storage bottle with information (preterm infant’s basic information, feeding volume per meal, feeding time, shelf life) or generating a QR code for portioning (24,48) | 5c | A |
| MOM heating | 63. Use a bottle warmer or warm water (≥37 but <40 ℃) for approximately 15 minutes. Do not use boiling water or a microwave (24,26) | 1c | A |
| 64. MOM stored in syringes should be placed in the neonatal incubator for gradual warming within 30 minutes before administration (24) | 5c | A | |
| Implementation of COIT | 65. Verify the information before each use of MOM by manual check or scanning a QR code to prevent errors (24,48) | 5c | A |
| 66. Initiate COIT within 24 postpartum hours, administering every two to four hours until partial oral feeding tolerance is established (45,48) | 5b | A | |
| 67. For preterm infants who meet enteral feeding standards, it is recommended to perform COIT five minutes before feeding (45) | 5c | B | |
| 68. Apply 0.2 mL of MOM with a sterile cotton swab to the oral mucosa, tongue, gums, and lips, or use a 1 mL sterile syringe to drop MOM onto the infant’s inner cheek (45) | 5b | A | |
COIT, colostrum oral immune therapy; ELBW, extremely low birth weight; LOS, late-onset sepsis; MOM, mother’s own milk; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; QR, quick response.
"V体育官网" Discussion
Training or educating stakeholders is a prerequisite for successful evidence application
MOM feeding support in the NICU is substantially more complex than in normal term infants, as relying solely on NICU efforts is insufficient. The basic team should include frontline clinical staff from neonatology and obstetrics, with optional invitations to lactation consultants, nutritionists, milk technicians (MT), and peer supporters (50,51). The training methods and programs should be reasonably designed according to institutional advantages (52).
Current breastfeeding education mainly focuses on the postpartum period. However, since adverse pregnancy outcomes are not always unpredictable, additional prepartum counseling for high-risk pregnant women and their families is crucial to enable informed decision-making rapidly after delivery (53). Educational materials ensure parents understand and follow MOM feeding protocols, and additional methods like consultation clinics (51) and online platforms (53) can enhance education.
In summary, both staff training and parental education should ensure that stakeholders are clear about the detailed processes of NICU’s early MOM application, enabling effective collaboration and ensuring the successful evidence implementation.
Milk expression and lactation support are foundation for early MOM application
Studies have shown that the lack of MOM is a major barrier to the early use of MOM in NICU (50). NICU infants’ mothers often exhibit multiple risk factors that contribute to delayed lactation. Therefore, it is particularly important to support mothers in early postpartum milk expression and promoting lactation after delivery. Whenever possible, antenatal education and training should be provided to mothers on lactation logs, hand milk expression, electric breast pump use, milk storage protocols, and NICU milk transportation procedures (24). Breast milk expression is recommended to initiate within the first 6 postpartum hours (46), which requires lactation support before mothers return to the obstetrics ward (11). Additionally, maintaining a frequency of at least 8 times a day presents a significant challenge for mothers and their families. Post discharge home-based breast milk expression for mothers often lacks timely professional guidance and procedural supervision, which may lead to insufficient lactation initiation and establishment (54). Therefore, evidence application should focus on lactation support strategies targeting NICU mothers.
Beyond fundamental education and technical support, targeted material and policy incentives—requiring higher-level administrative backing—are essential. For instance, the U.S. Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) provides postpartum women with free medical-grade breast pumps (24). However, due to economic constraints, developing countries such as China lack policies ensuring affordable access to such equipment, potentially compromising early lactation support. When feasible, mothers should be provided with infant photographs or involved in basic care, including Kangaroo Mother Care (KMC), to promote family engagement and mother-infant bonding (52). However, widespread adoption of these practices demands substantial economic and resource investment, currently limited to a few hospitals in major Chinese cities such as Beijing and Shanghai. Nevertheless, all institutions should prioritize providing mothers with essential lactation guidance to support early initiation of MOM.
Strictly follow the standards for MOM storage, transportation, and handling to ensure safety (V体育2025版)
Due to maternal-infant separation, MOM must undergo multiple procedures before administration to preterm infants. Regarding milk storage, included literature specify the containers, temperature and corresponding time, but there are some variations in the required conditions. For example, the expert consensus recommends using refrigerated breast milk within 72 hours (48), while clinical guidelines suggest up to 96 hours (24). Breast milk transport requires a cold-chain, with dry ice recommended. However, dry ice use is costly, and no high-quality studies validate whether an 18-hour threshold is appropriate for its application. In the comprehensive management of MOM, the literature recommends implementing electronic information management (24,48), and establishing milk delivery-reception-portioning codes to further ensure feeding safety. In summary, the evidence on MOM management primarily comes from guidelines or consensus on milk bank management, which are based on donated milk practices, while targeted research on MOM management is still lacking.
A select group of investigators has recognized the imperative for optimized MOM processing protocols, initiating feasibility-driven initiatives tailored to their healthcare institutions’ operational frameworks. For instance, two NICUs in the United States pioneered the integration of electronic health records (EHRs) to enable real-time tracking of individual MOM units. This system facilitates data-driven workflow rationalization for clinical staff (13,50). Nevertheless, multifactorial barriers—including development costs, system maintenance, and staffing constraints—sustain manual supervision protocols for MOM management in Chinese NICUs (51). Accordingly, implementing EHR-enabled MOM administration systems necessitates pragmatic investigation by researchers to establish contextually viable operational frameworks.
Clarify the benefits, implementation methods, and challenges of COIT (VSports在线直播)
Although some NICU preterm infants cannot be fed orally early on, COIT can stimulate oropharyngeal-associated lymphoid tissue (OFALT) to provide MOM’s anti-inflammatory and anti-allergic protection. This study has incorporated multiple systematic reviews that demonstrate the safety of COIT and its beneficial effects on preterm infants’ outcomes. Evidence suggests that COIT should be implemented within 24 hours of birth, ensuring the early application of MOM. Furthermore, the prerequisite requirement for MOM availability constrains COIT implementation. For institutions not yet implementing COIT, its introduction imposes additional clinical workload. Consequently, workforce engagement initiatives become critical components for successfully integrating COIT-derived innovations into routine practice (52).
It is important to note that there is no unified standard for the implementation of COIT. Additionally, the conclusions of the included systematic reviews mainly focus on the impact of COIT on outcome-related indicators for NICU infants, with no critical appraisal of how implementation heterogeneity modulates intervention efficacy. Secondly, in the systematic reviews involving COIT, the populations predominantly comprised infants with very low or extremely low birth weight, as well as those born very or extremely preterm (4,27,30-33,35-37,39,40,43). For preterm infants with birth weight exceeding 1,500 g or gestational age beyond 32 weeks, the clinical efficacy and necessity of COIT implementation remain unexamined in extant literature.
Conclusions
This study systematically retrieved the evidence on improving the early use of MOM in NICU preterm infants with COIT, synthesizing 68 evidence items across eight categories. This study only included publicly published Chinese and English literature, which may lead to incomplete evidence inclusion, and the evidence regarding the implementation protocol primarily comes from Chinese literature. Especially in MOM’s standardized management, most of the evidence comes from literature on milk bank management in various countries. Due to the current lack of uniform practices in original research regarding the management of MOM for preterm infants and the implementation of COIT, such literature was not included during the literature search and screening process. Consequently, this analysis exclusively incorporates secondary evidence. Future original research should focus on the question of ‘which approach is more effective and efficient’ through high-quality RCTs. The implementation tools of COIT, single-dose MOM usage, start time, frequency of implementation, and cutoff time, as well as their various combinations, have not been thoroughly analyzed in terms of their impact on clinical outcomes in preterm infants. Currently, only one registered study protocol indicates nascent scholarly attention to this clinical knowledge gap (55).
In the process of clinical translation of evidence, appropriate adjustment and application of each piece of evidence should be made based on its FAME attributes. Optimally, future research should translate existing evidence into clinical practice through various theories, particularly well-established implementation science models such as the Consolidated Framework for Implementation Research (CFIR) (56) and the Behavior Change Wheel (BCW) (57). These theoretically anchored approaches enable methodologically rigorous integration of evidence into routine clinical practice.
Acknowledgments
None.
"VSports注册入口" Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-245/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-245/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-245/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
VSports - References
- UNICEF. 150 million babies born preterm in the last decade. 09 May 2023. Available online: https://www.unicef.org/press-releases/150-million-babies-born-preterm-last-decade
- Sokou R, Parastatidou S, Ioakeimidis G, et al. Breastfeeding in Neonates Admitted to an NICU: 18-Month Follow-Up. Nutrients 2022;14:3841. [Crossref] [PubMed]
- Ohuma EO, Moller AB, Bradley E, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet 2023;402:1261-71. [Crossref (VSports手机版)] [PubMed]
- Fu ZY, Huang C, Lei L, et al. The effect of oropharyngeal colostrum administration on the clinical outcomes of premature infants: A meta-analysis. Int J Nurs Stud 2023;144:104527. [Crossref] [PubMed]
- Quitadamo PA, Zambianco F, Palumbo G, et al. Trend and Predictors of Breastmilk Feeding among Very-Low-Birth-Weight Infants in NICU and at Discharge. Nutrients 2023;15:3314. [Crossref] [PubMed]
- Meier PP. More evidence: Mothers’ own milk is personalized medicine for very low birthweight infants. Cell Rep Med 2022;3:100710. [Crossref] [PubMed]
- Hilditch C, Rumbold AR, Keir A, et al. Effect of Neonatal Unit Interventions Designed to Increase Breastfeeding in Preterm Infants: An Overview of Systematic Reviews. Neonatology 2024;121:411-20. [Crossref] [PubMed]
- Parker MG, Stellwagen LM, Noble L, et al. Promoting Human Milk and Breastfeeding for the Very Low Birth Weight Infant. Pediatrics 2021;148:e2021054272. [Crossref] [PubMed]
- Tomlinson C, Haiek LN. Breastfeeding and human milk in the NICU: From birth to discharge. Paediatr Child Health 2023;28:510-26. [Crossref] [PubMed]
- Rodriguez NA, Meier PP, Groer MW, et al. Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. J Perinatol 2009;29:1-7. [Crossref (V体育2025版)] [PubMed]
- Balasundaram M, Land R, Miller S, et al. Increasing early exposure to mother's own milk in premature newborns. J Perinatol 2022;42:1126-34. [V体育2025版 - Crossref] [PubMed]
- Digal KC, Upadhyay J, Singh P, et al. Oral Care with Mother’s Own Milk in Sick and Preterm Neonates: A Quality Improvement Initiative. Indian J Pediatr 2021;88:50-7. ["VSports注册入口" Crossref] [PubMed]
- Wetzel CM, Davis L, Grohler N, et al. A Quality Improvement Project to Improve the Use of Mother's Own Milk (MOM) With Precision Oropharyngeal Therapy. Adv Neonatal Care 2020;20:E19-E30. ["VSports注册入口" Crossref] [PubMed]
- Zhu Z, Hu Y, Zhou YF, et al. Promoting evidence translation into clinical practice (Part 3): Research topic selection and question formulation. J Nurs Train 2020;35:796-9.
- Dicenso A, Bayley L, Haynes RB. Accessing pre-appraised evidence: fine-tuning the 5S model into a 6S model. Evid Based Nurs 2009;12:99-101. [Crossref (VSports手机版)] [PubMed]
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [Crossref] [PubMed]
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839-42. [Crossref] [PubMed]
- Zhu Z, Hu Y, Zhou YF, et al. Promoting evidence translation into clinical practice (Part 5): Literature quality appraisal in translational research. J Nurs Train. 2020;35:996-1000.
- Foster MJ, Shurtz S. Making the Critical Appraisal for Summaries of Evidence (CASE) for evidence-based medicine (EBM): critical appraisal of summaries of evidence. J Med Libr Assoc 2013;101:192-8. [Crossref] [PubMed]
- Hu Y, Hao YF. Evidence-Based Nursing. 2nd ed. Beijing: People’s Medical Publishing House; 2018:45-50.
- Wang CQ, Hu Y. JBI evidence pre-grading and evidence recommendation hierarchy system (version 2014). J Nurs Train 2015;30:964-7.
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. JBI Levels of Evidence. October 2013. Available online: https://jbi.global/sites/default/files/2019-05/JBI-Levels-of-evidence_2014_0.pdf
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. JBI Grades of Recommendation. October 2013. Available online: https://jbi.global/sites/default/files/2019-05/JBI-grades-of-recommendation_2014.pdf
- Copertino R, Parker MG. Breast milk expression for the preterm infant. UpToDate 2025. Available online: https://sy.hbwuganfu.com/contents/breast-milk-expression-for-the-preterm-infant
- Copertino R, Parker MG. Breastfeeding the preterm infant. UpToDate 2025. Available online: https://sy.hbwuganfu.com/contents/breastfeeding-the-preterm-infant
- Yang PY, Shi SP, Zhang YX, et al. Adaptation and appraisal of evidence-based guidelines for breastfeeding in hospitalized neonates. Chin J Nurs 2018;53:57-64.
- Anne RP, Kumar J, Kumar P, et al. Effect of oropharyngeal colostrum therapy on neonatal sepsis in preterm neonates: A systematic review and meta-analysis. J Pediatr Gastroenterol Nutr 2024;78:471-87. [Crossref] [PubMed]
- Gomez JA, Abela K, LoBiondo-Wood G. A Systemic Review of the Difference Between Diets for Preterm Infants Containing Raw Mother's Own Milk and Frozen or Pasteurized Mother's Own Milk. J Hum Lact 2024;40:259-69. [V体育ios版 - Crossref] [PubMed]
- Lang Y, Jiang XX, Yang Z, et al. Meta-analysis of colostrum oral immunotherapy for preventing late-onset sepsis in preterm infants. Wuhan Univ J Med Sci 2023;44:1027-32.
- Kumar J, Meena J, Ranjan A, et al. Oropharyngeal application of colostrum or mother's own milk in preterm infants: a systematic review and meta-analysis. Nutr Rev 2023;81:1254-66. [Crossref] [PubMed]
- Cai M, Lin L, Peng Y, et al. Effect of Breast Milk Oral Care on Mechanically Ventilated Preterm Infants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Pediatr 2022;10:899193. ["V体育官网入口" Crossref] [PubMed]
- Huo M, Liu C, Mei H, et al. Intervention Effect of Oropharyngeal Administration of Colostrum in Preterm Infants: A Meta-Analysis. Front Pediatr 2022;10:895375. ["V体育安卓版" Crossref] [PubMed]
- Zhang XY, Liu J, Mu XH, et al. Meta-analysis of colostrum oral immunotherapy for improving gastrointestinal feeding outcomes in preterm infants. J Evid Based Nurs 2022;8:1847-53.
- Zhou WJ, Chen L, Yan FF, et al. Meta-analysis of sterilization methods and efficacy for cytomegalovirus-infected breast milk. Chin J Disinfect 2022;39:756-62.
- Xavier Ramos MS, Martins CDC, Souza ES, et al. Oropharyngeal colostrum immunotherapy and nutrition in preterm newborns: meta-analysis. Rev Saude Publica 2021;55:59. [Crossref] [PubMed]
- Cai HW, Ma YL, Liu YS, et al. Meta-analysis of oral immunotherapy for preventing ventilator-associated pneumonia in preterm infants. Chin J Mod Nurs 2021;27:2563-9.
- Tao J, Mao J, Yang J, et al. Effects of oropharyngeal administration of colostrum on the incidence of necrotizing enterocolitis, late-onset sepsis, and death in preterm infants: a meta-analysis of RCTs. Eur J Clin Nutr 2020;74:1122-31. [Crossref] [PubMed]
- Panchal H, Athalye-Jape G, Patole S. Oropharyngeal Colostrum for Preterm Infants: A Systematic Review and Meta-Analysis. Adv Nutr 2019;10:1152-62. [Crossref] [PubMed]
- Li YY, Zhao X, Li GZ, et al. Systematic review of oral care interventions using colostrum in preterm infants. Chin J Nurs 2019;54:753-9.
- Wang Q, Zhang XH, Wei L, et al. Meta-analysis of colostrum oral swabbing/dripping for preventing necrotizing enterocolitis in preterm infants. J Clin Pediatr 2019;37:712-7.
- Garg BD, Balasubramanian H, Kabra NS, et al. Effect of oropharyngeal colostrum therapy in the prevention of necrotising enterocolitis among very low birthweight neonates: A meta-analysis of randomised controlled trials. J Hum Nutr Diet 2018;31:612-24. [Crossref] [PubMed]
- Nasuf AWA, Ojha S, Dorling J. Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev 2018;9:CD011921. [Crossref] [PubMed]
- Xu SH, Zhang QM, Dan X, et al. Meta-analysis of oral immunotherapy efficacy in preterm infants. Chin Nurs Manag 2018;18:1340-6.
- Becker GE, Smith HA, Cooney F. Methods of milk expression for lactating women. Cochrane Database Syst Rev 2016;9:CD006170. [Crossref] [PubMed]
- Ji RT, Lin DN, Liu XC, et al. Best evidence summary of colostrum oral immunotherapy for preterm infants. J Nurs (China) 2024;31:37-42.
- Fu ZY, Zhang X, Hu Y, et al. Best evidence summary of lactation maintenance during mother-infant separation in mothers of very low birth weight infants. Chin J Pract Nurs 2021;37:18-25.
- Jin YM, Gu WW, Yang HH, et al. Best evidence summary of breast milk collection for mothers of separated preterm infants. Chin J Mod Nurs 2020;26:421-8.
- Cao Y, Li ZH, Han SP, et al. Expert consensus on breast milk use in neonatal intensive care units. Chin J Evid Based Pediatr 2021;16:171-8.
- Perinatal Medicine Branch of Chinese Medical Association. Expert consensus on common maternal infections and breastfeeding guidance. Chin J Perinat Med 2021;24:481-9.
- Fleiss N, Morrison C, Nascimento A, et al. Improving Early Colostrum Administration to Very Low Birth Weight Infants in a Level 3 Neonatal Intensive Care Unit: A Quality Improvement Initiative. J Pediatr 2023;260:113421. [Crossref] [PubMed]
- Hu XJ, Zhang YX, Li LL, et al. Development of breastfeeding models for very low birth weight preterm infants during NICU hospitalization. Chin Nurs Manag 2020;20:14-7.
- Swanson NM, Elgersma KM, McKechnie AC, et al. Encourage, Assess, Transition (EAT): A Quality Improvement Project Implementing a Direct Breastfeeding Protocol for Preterm Hospitalized Infants. Adv Neonatal Care 2023;23:107-19. [Crossref] [PubMed]
- Flynn KE, McDonnell SM, Brazauskas R, et al. Smartphone-Based Video Antenatal Preterm Birth Education: The Preemie Prep for Parents Randomized Clinical Trial. JAMA Pediatr 2023; Epub ahead of print. [Crossref]
- Jiang X, Jiang H. Factors associated with post NICU discharge exclusive breastfeeding rate and duration amongst first time mothers of preterm infants in Shanghai: a longitudinal cohort study. Int Breastfeed J 2022;17:34. [Crossref (V体育官网入口)] [PubMed]
- Jin Y, Fu Z, Jin J, et al. Varying oropharyngeal colostrum administration dosages on outcomes associated with very low birth weight infants: a randomised controlled trial protocol. BMJ Open 2025;15:e097496. [Crossref] [PubMed]
- Zhang Q, Han S, Dong W, et al. Barriers and facilitators to the application of nurse practitioners' training pilot programs in China: A CFIR-guided descriptive qualitative study. Nurse Educ Today 2025;145:106501. [Crossref] [PubMed]
- Couto N, Morgado V, Pereira T, et al. Behavior change wheel as a tool to promote physical activity in online intervention: a case study. Front Psychol 2025;16:1498351. ["VSports最新版本" Crossref] [PubMed]