Identification of Frataxin as a regulator of ferroptosis
- PMID: 32169822
- PMCID: PMC7068686
- DOI: 10.1016/j.redox.2020.101483 (VSports app下载)
Identification of Frataxin as a regulator of ferroptosis
Erratum in (VSports)
-
Corrigendum to "Identification of Frataxin as a regulator of ferroptosis" [Redox Biol. 32 (2020) 101483].Redox Biol. 2023 Sep;65:102815. doi: 10.1016/j.redox.2023.102815. Epub 2023 Jul 28. Redox Biol. 2023. PMID: 37517878 Free PMC article. No abstract available.
"VSports在线直播" Abstract
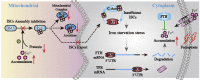
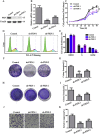
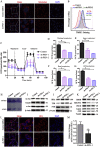
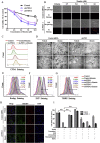
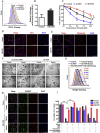
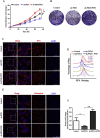
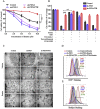
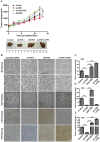
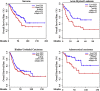
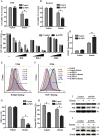
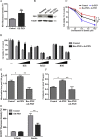
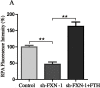
Ferroptosis is a newly discovered form of non-apoptotic regulated cell death and is characterized by iron-dependent and lipid peroxidation. Due to the enhanced dependence on iron in cancer cells, induction of ferroptosis is becoming a promising therapeutic strategy VSports手机版. However, the precise underlying molecular mechanism and regulation process of ferroptosis remains largely unknown. In the present study, we demonstrate that the protein Frataxin (FXN) is a key regulator of ferroptosis by modulating iron homeostasis and mitochondrial function. Suppression of FXN expression specifically repressed the proliferation, destroyed mitochondrial morphology, impeded Fe-S cluster assembly and activated iron starvation stress. Moreover, suppression of FXN expression significantly enhanced erastin-induced cell death through accelerating free iron accumulation, lipid peroxidation and resulted in dramatic mitochondria morphological damage including enhanced fragmentation and vanished cristae. In addition, this type of cell death was confirmed to be ferroptosis, since it could be pharmacologically restored by ferroptotic inhibitor Fer-1 or GSH, but not by inhibitors of apoptosis, necrosis. Vice versa, enforced expression of FXN blocked iron starvation response and erastin-induced ferroptosis. More importantly, pharmacological or genetic blocking the signal of iron starvation could completely restore the resistance to ferroptosis in FXN knockdown cells and xenograft graft in vivo. This paper suggests that FXN is a novel ferroptosis modulator, as well as a potential provided target to improve the antitumor activity based on ferroptosis. .
Keywords: Ferroptosis; Frataxin; Iron-sulfur cluster; Mitochondria V体育安卓版. .
Copyright © 2020 The Authors. Published by Elsevier B V体育ios版. V. All rights reserved. .
Conflict of interest statement
Declaration of competing interest The authors declare that there is no conflict of interest.
"V体育安卓版" Figures

References
-
- Puig S., Ramos-Alonso L., Romero A.M., Martinez-Pastor M.T. The elemental role of iron in DNA synthesis and repair. Metallomics. 2017;9:1483–1500. - PubMed
-
- Furuyama K., Kaneko K. Iron metabolism in erythroid cells and patients with congenital sideroblastic anemia. Int. J. Hematol. 2018;107:44–54. - PubMed (VSports)
-
- Dixon Scott J., Lemberg Kathryn M., Lamprecht Michael R., Skouta R., Zaitsev Eleina M., Gleason Caroline E., Patel Darpan N., Bauer Andras J., Cantley Alexandra M., Yang Wan S. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. - V体育ios版 - PMC - PubMed
-
- A P., Fl I., Jm M., Pj C. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019;96:291–301. - PubMed
Publication types
"V体育安卓版" MeSH terms
- "V体育官网入口" Actions
- "V体育安卓版" Actions
- Actions (VSports)
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

