"VSports注册入口" Reprogramming of stromal fibroblasts by SNAI2 contributes to tumor desmoplasia and ovarian cancer progression
- PMID: 29041931
- PMCID: PMC5645935
- DOI: 10.1186/s12943-017-0732-6
"VSports" Reprogramming of stromal fibroblasts by SNAI2 contributes to tumor desmoplasia and ovarian cancer progression
Abstract
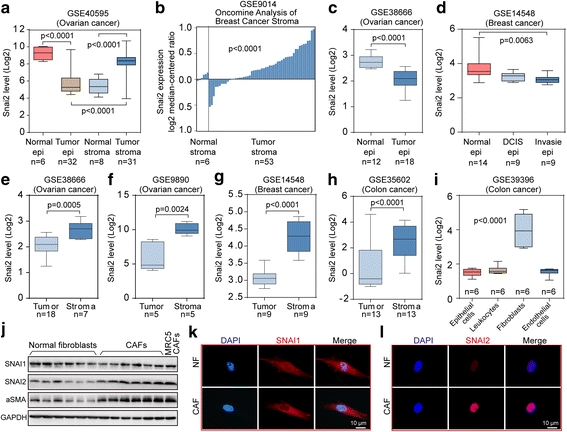
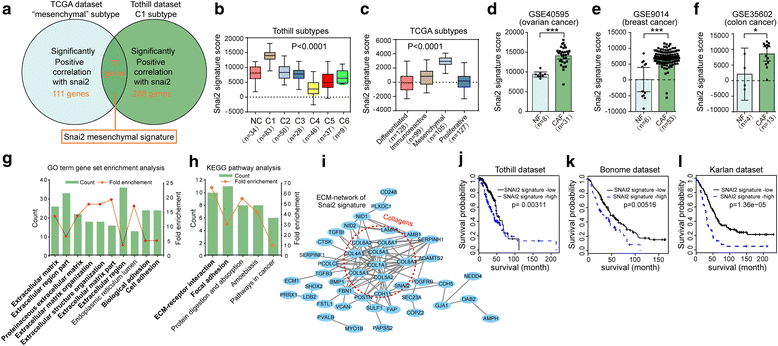
Background: Molecular profiling in ovarian cancer (OC) revealed that the desmoplasia subtype presented the poorest prognosis, highlighting the contribution of stromal fibroblasts in tumor progression. This study aimed to investigate the molecular characteristics of SNAI2 driving the transcriptional reprogramming of fibroblasts within tumors. VSports手机版.
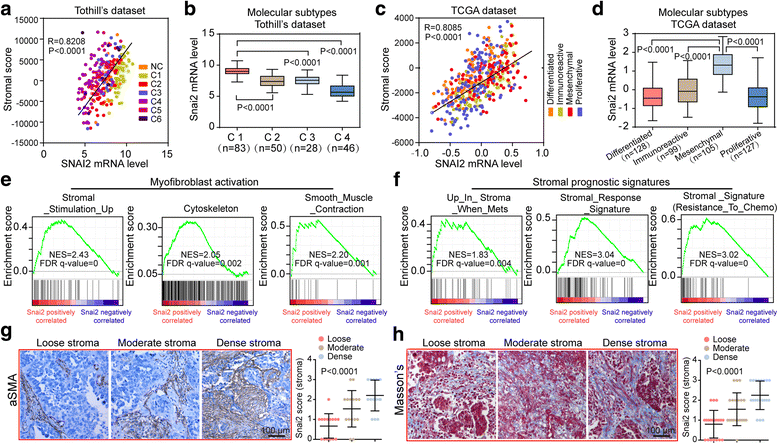
Methods: SNAI2 expression was evaluated in microdissected profiles of various cancers and in various molecular subtypes of OC. Gene set enrichment analysis (GSEA) and single sample GSEA (ssGSEA) were performed to explore the correlation between SNAI2 and stromal fibroblast activation. The SNAI2 defined signature in the mesenchymal OC subtype was identified through an integrative analysis of the TCGA and the Tothill datasets. The predictive value of this signature was validated in independent datasets V体育安卓版. SNAI2 expression alteration influence of tumor growth in primary CAFs was evaluated in 3D organotypic and murine xenograft models. .
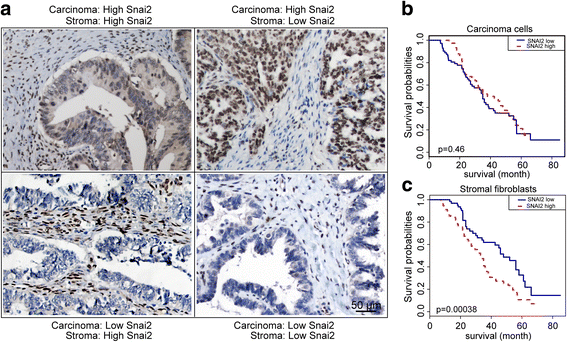
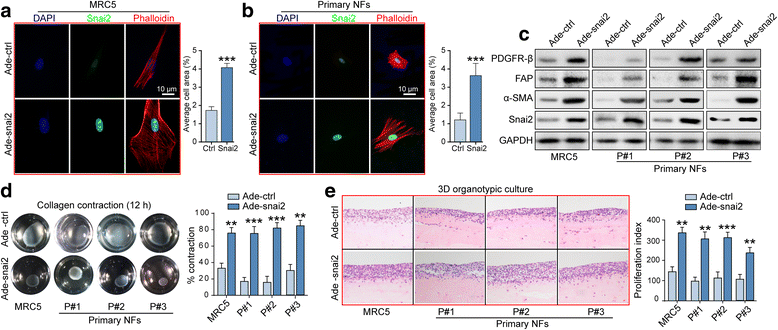
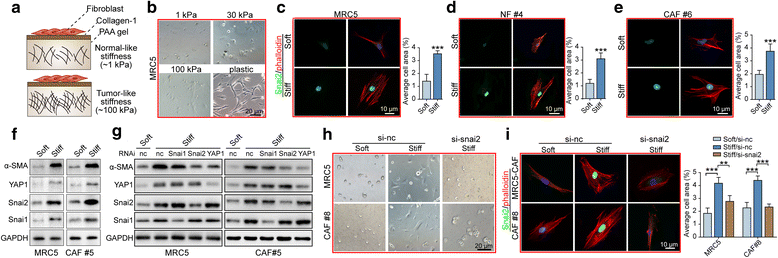
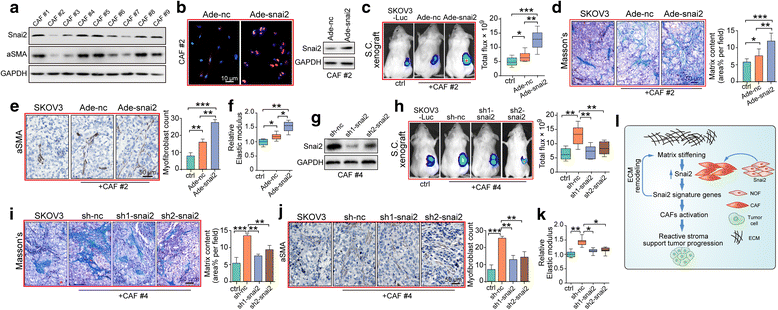
Results: We demonstrated that SNAI2 was frequently activated in the tumor stroma, correlated with fibroblast activation and worse patient outcome in OC. SNAI2 transformed normal fibroblasts to a CAF-like state and boosted their tumor-supporting role in 3D organotypic culture and in OC xenograft model V体育ios版. SNAI2 drove a transcriptional signature in the mesenchymal subtype of OC that contributed to tumor desmoplasia, which fed back to increase SNAI2 expression and sustain fibroblast activation. .
Conclusions: Our results address the role of SNAI2 in reprogramming stromal fibroblasts VSports最新版本. The identified SNAI2 mesenchymal signature has both a predictive value and biological relevance and might be a therapeutic target for stroma-oriented therapy against the desmoplasia OC subtype. .
Keywords: Ovarian cancer; Reprogram; SNAI2; Stiffness; Stromal fibroblast V体育平台登录. .
VSports在线直播 - Conflict of interest statement
Ethics approval and consent to participate
All participants provided written informed consent, and the study was approved by the Institutional Research Ethic Committee of Tongji hospital, Huazhong University of Science and Technology.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
V体育2025版 - References
-
- De Sousa E, Melo F, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, de Jong JH, de Boer OJ, van Leersum R, Bijlsma MF, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–618. doi: 10.1038/nm.3174. - DOI (V体育官网) - PubMed
-
- Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro B, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. - DOI - PubMed
Publication types
MeSH terms
- "V体育官网" Actions
- Actions (V体育官网)
- "V体育ios版" Actions
Substances
- Actions (V体育平台登录)
- VSports注册入口 - Actions
LinkOut - more resources
Full Text Sources (V体育平台登录)
Other Literature Sources
Medical
"V体育官网入口" Research Materials

