CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism
- PMID: 27304511
- PMCID: VSports在线直播 - PMC4911708
- DOI: 10.1016/j.cmet.2016.05.006
CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism
Abstract
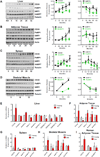
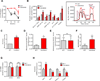
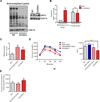
Nicotinamide adenine dinucleotide (NAD) levels decrease during aging and are involved in age-related metabolic decline. To date, the mechanism responsible for the age-related reduction in NAD has not been elucidated. Here we demonstrate that expression and activity of the NADase CD38 increase with aging and that CD38 is required for the age-related NAD decline and mitochondrial dysfunction via a pathway mediated at least in part by regulation of SIRT3 activity. We also identified CD38 as the main enzyme involved in the degradation of the NAD precursor nicotinamide mononucleotide (NMN) in vivo, indicating that CD38 has a key role in the modulation of NAD-replacement therapy for aging and metabolic diseases VSports手机版. .
Keywords: CD38; NAD(+); aging; glucose intolerance; mitochondrial function. V体育安卓版.
Copyright © 2016 Elsevier Inc V体育ios版. All rights reserved. .
Figures



"VSports" References
-
- Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 2006;345:1386–1392. - PubMed
-
- Bai P, Cantó C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012;16:290–295. - PubMed
-
- Bakondi E, Catalgol B, Bak I, Jung T, Bozaykut P, Bayramicli M, Ozer NK, Grune T. Age-related loss of stress-induced nuclear proteasome activation is due to low PARP-1 activity. Free Radic. Biol. Med. 2011;50:86–92. - PubMed
-
- Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, Feitoza S, Sieck G, Chini EN. The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology. 2004;145:881–889. - PubMed (VSports手机版)
"VSports" MeSH terms
- "VSports app下载" Actions
- "V体育平台登录" Actions
- Actions (VSports注册入口)
- "V体育安卓版" Actions
- "VSports" Actions
- VSports注册入口 - Actions
- VSports最新版本 - Actions
- V体育2025版 - Actions
- V体育平台登录 - Actions
Substances
- Actions (VSports手机版)
- Actions (VSports app下载)
- "VSports app下载" Actions
- Actions (V体育安卓版)
Grants and funding
V体育官网 - LinkOut - more resources
Full Text Sources (VSports)
"V体育2025版" Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

