Identification of diverse lipid droplet targeting motifs in the PNPLA family of triglyceride lipases
- PMID: 23741432
- PMCID: V体育官网入口 - PMC3669214
- DOI: "VSports注册入口" 10.1371/journal.pone.0064950
Identification of diverse lipid droplet targeting motifs in the PNPLA family of triglyceride lipases
Abstract
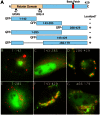
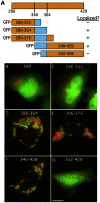
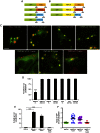
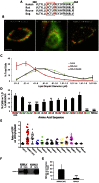
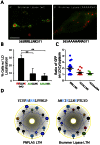
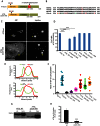
Members of the Patatin-like Phospholipase Domain containing Protein A (PNPLA) family play key roles in triglyceride hydrolysis, energy metabolism, and lipid droplet (LD) homoeostasis VSports手机版. Here we report the identification of two distinct LD targeting motifs (LTM) for PNPLA family members. Transient transfection of truncated versions of human adipose triglyceride lipase (ATGL, also known as PNPLA2), PNPLA3/adiponutrin, or PNPLA5 (GS2-like) fused to GFP revealed that the C-terminal third of these proteins contains sequences that are sufficient for targeting to LDs. Furthermore, fusing the C-termini of PNPLA3 or PNPLA5 confers LD localization to PNPLA4, which is otherwise cytoplasmic. Analyses of additional mutants in ATGL, PNPLA5, and Brummer Lipase, the Drosophila homolog of mammalian ATGL, identified two different types of LTMs. The first type, in PNPLA5 and Brummer lipase, is a set of loosely conserved basic residues, while the second type, in ATGL, is contained within a stretch of hydrophobic residues. These results show that even closely related members of the PNPLA family employ different molecular motifs to associate with LDs. .
Conflict of interest statement
Figures
References
-
- Lass A, Zimmermann R, Oberer M, Zechner R (2011) Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 50: 14–27. - V体育官网 - PMC - PubMed
"V体育官网入口" Publication types
MeSH terms
- "VSports" Actions
- V体育ios版 - Actions
- Actions (VSports最新版本)
- Actions (V体育安卓版)
- "V体育平台登录" Actions
- VSports在线直播 - Actions
- "VSports" Actions
- VSports在线直播 - Actions
- VSports手机版 - Actions
V体育ios版 - Substances
- V体育平台登录 - Actions
"V体育官网" Grants and funding
LinkOut - more resources (V体育官网入口)
Full Text Sources
"VSports app下载" Other Literature Sources
Molecular Biology Databases
Research Materials

