Summary
Background
Childhood-onset combined pituitary hormone deficiency (CPHD) has a wide spectrum of etiologies and genetic causes for congenital disease VSports注册入口. We aimed to describe the clinical spectrum and genetic etiologies of CPHD in a single tertiary center and estimate the population-level incidence of congenital CPHD.
V体育官网入口 - Methods
The retrospective clinical cohort comprised 124 CPHD patients (48 with congenital CPHD) treated at the Helsinki University Hospital (HUH) Children's Hospital between 1985 and 2018. Clinical data were collected from the patient charts. Whole exome sequencing was performed in 21 patients with congenital CPHD of unknown etiology V体育官网入口.
Findings
The majority (61%;76/124) of the patients had acquired CPHD, most frequently due to craniopharyngiomas and gliomas. The estimated incidence of congenital CPHD was 1/16 000 (95%CI, 1/11 000-1/24 000). The clinical presentation of congenital CPHD in infancy included prolonged/severe neonatal hypoglycaemia, prolonged jaundice, and/or micropenis/bilateral cryptorchidism in 23 (66%) patients; despite these clinical cues, only 76% of them were referred to endocrine investigations during the first year of life. The median delay between the first violation of the growth screening rules and the initiation of GH Rx treatment among all congenital CPHD patients was 2·2 years, interquartile range 1·2–3·7 years. Seven patients harbored pathogenic variants in PROP1, SOX3, TBC1D32, OTX2, and SOX2, and one patient carried a likely pathogenic variant in SHH (c VSports在线直播. 676G>A, p. (Ala226Thr)).
Interpretation
Our study suggests that congenital CPHD can occur in 1/16 000 children, and that patients frequently exhibit neonatal cues of hypopituitarism and early height growth deflection V体育2025版. These results need to be corroborated in future studies and might inform clinical practice.
Funding
Päivikki and Sakari Sohlberg Foundation, Biomedicum Helsinki Foundation, and Emil Aaltonen Foundation research grants.
Keywords: Hypopituitarism, CPHD, Incidence, Growth sreening, Hypopituitarism etiology, Hypopituitarism genetic
Research in context.
"VSports注册入口" Evidence before this study
Childhood-onset combined pituitary hormone deficiency (CPHD) is a multifaceted diagnosis covering multiple congenital and acquired etiologies with variable diagnostic aspects V体育官网. In addition to our earlier literature reviews, we searched PubMed for several CPHD-related terms, including “childhood hypopituitarism”, “childhood CPHD”, “CPHD incidence”, “childhood hypopituitarism incidence”, and “idiopathic hypopituitarism” (altogether 1176 results). We were able to identify four previous studies that described the entire spectrum of CPHD, only one of which was conducted in pediatric patients in the developing economy of Sudan, with a high rate of consanguinity. Untreated CPHD is a life-threatening disease due to the lack of cortisol responses to stress, and later in childhood, it may compromise growth and development. This highlights the importance of early diagnosis and treatment of CPHD. During the last few years, multiple new candidate genes have been implicated in congenital CPHD. However, their significance has not been examined in larger patient cohorts.
Added value of this study
We present to the best of our knowledge the first incidence estimates for congenital CPHD derived from a cohort of carefully documented patients with two or more pituitary hormone deficiencies. We describe presentation and diagnostic features of pediatric CPHD VSports手机版. We suggest the benefits of children's growth monitoring for the early referral and we investigate the presence of rare sequence variants in a set of 80 genes implicated in CPHD in 21 patients with congenital CPHD.
Implications of all the available evidence
Our study and the previous studies congruently underline the diagnostic challenges of the rare but life-threatening CPHD. We provide understanding on the diagnostic cues of the disease, including the potential of population-based growth screening and the frequent presence of neonatal features of CPHD (prolonged hypoglycaemia, jaundice, and micropenis/cryptorchidism in males). Future research should consider means to reduce the diagnostic delay of the CPHD patients, including amendments to the neonatal screening and the possibilities to strengthen the identification of neonatal cues and growth retardation.
Alt-text: Unlabelled box
"VSports" Introduction
The hypothalamic-pituitary axis is an essential regulator of somatic growth, puberty, metabolism, neuro-behavioral development, and vital body stress responses. In patients with combined pituitary hormone deficiency (CPHD), i.e., deficiency of more than one pituitary hormone (adrenocorticotropin (ACTH), growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyrotropin (TSH), and antidiuretic hormone (ADH)), an early diagnosis is essential to ascertain timely growth and development, and to avoid life-threatening consequences of hypocortisolism resulting from ACTH deficiency.1
CPHD has multiple etiologies. Acquired CPHD may develop secondary to pituitary and non-pituitary tumors and their treatment, brain damage, infarction, autoimmune disorders, infiltrative diseases, infections, paraneoplastic syndromes, or as a side effect of drug therapy. In congenital CPHD, the hypothalamo-pituitary function is compromised due to developmental defects of the brain, or disruption of the pituitary hormone secretion of genetic/unidentified etiology. Congenital hypopituitarism may be evident at birth, or of evolving nature and manifest later during childhood. Syndromic features may guide the diagnosis of congenital CPHD, as can the symptoms and investigations of the underlying disease in patients with acquired CPHD. However, both congenital and acquired CPHD may present with similar endocrine problems.1, 2, 3 Few studies describe the etiologic spectrum of CPHD including both the acquired and congenital etiologies, and these have mainly been conducted in adult patients, except for one paper on hypopituitarism in Sudanese children.4, 5, 6
Of the known genetic defects causing CPHD, pathogenic variants in PROP1 are most common, although high variation exists in the reported frequency in different populations. Pathogenic variants in PROP1 have been identified in 0-64·8% of patients in different CPHD series with nationalities such as Lithuanian, Russian, Hungarian, Portuguese, Czech, and Brazilian harboring them significantly more often than the others.7, 8, 9 Other vital pituitary developmental genes that are implicated in CPHD include GLI2, HESX1, LHX3, LHX4, OTX2, POU1F1, SOX2, and SOX3, but the list is expanding rapidly. In total, at least 33 known CPHD genes have been reported to date.1,3,10, 11, 12 We searched the literature for CPHD/growth hormone deficiency-associated genes and were able to list 80 different genes (Supplementary Table 1), also including those with variants reported in, e.g., exome sequencing studies without further analyses of pathogenicity. Frequencies of pathogenic variants in well-established CPHD genes have been investigated in multiple patient series,7, 8, 9,13, 14, 15, 16, 17, 18, 19 whereas variants in the more recently identified genes implicated in CPHD have often been detected in only a few single cases. (see, e.g.,10,11,20, 21, 22, 23) Furthermore, few studies have examined all patients in a cohort for variants in all genes associated with CPHD. Thus, frequency estimates of the identified variants, especially in genes implicated in CPHD and identified only recently, might be biased.10 The majority (∼84% [45–100% in 21 studies], reviewed in Fang et al. 2016) of patients with congenital disease, including familial cases, remain without a genetic diagnosis, suggesting potentially unraveled genetic CPHD.3,7
With growth hormone deficiency (GHD) as a major component of CPHD, growth retardation is an important cue to an early diagnosis of the disease. Indeed, GHD with pituitary stalk interruption syndrome has been listed as a priority condition for growth monitoring, where substantial benefit could be achieved from the early detection of abnormal growth.24 In the light of previous studies on the early growth of patients with GHD, the height growth of GHD patients is reduced during the first year of life.25,26 In Finland, children's growth is monitored in well-child clinics, where over 99% of all children participate in regular follow-up measurements during childhood.27,28 The auxological data are subjected to growth screening rules, and children with divergent growth are referred to secondary health care.29, 30, 31, 32, 33, 34 The Finnish growth screening could be an effective tool for the early identification of patients with CPHD.29,31
We investigated the diagnostic spectrum and genetic features of childhood-onset CPHD in Finland's largest tertiary center between 1985 and 2018. We describe the etiologies, diagnostic features, and growth of the patients with congenital CPHD, to provide an understanding of the spectrum of childhood-onset CPHD, and the key drivers of early diagnosis. Furthermore, we evaluate the role of growth screening in facilitating early diagnosis of CPHD. Patients with CPHD of unknown etiology were investigated through whole exome sequencing for variants in the 80 identified CPHD/GHD-associated genes.
VSports app下载 - Methods
"VSports app下载" Patients
The formation of the patient cohort is shown in Figure 1. We searched the patient registry of Children and Adolescents at Helsinki University Hospital (HUH) for patients with diagnoses of hypopituitarism and related disorders from January 1985 to September 2018 (n=2718, Figure 1). From the search, we identified (i) hits for CPHD ICD-codes (n=237), (ii) familial cases (n=106), and (iii) hits for other hypopituitarism ICD-codes between January 2009 and September 2018 (from the launch of electronic patient records; n=644). Additionally, (iv) we went through growth database search results for HUH catchment area short patients (height ≤-3 SDS at the age of ≥3 years) between 1990 and 2015 (n=785).35 Hits (ii)-(iv) were inspected manually, to identify patients with CPHD but lacking the appropriate diagnosis. Patients with isolated hormone deficiencies, including IGHD, were excluded, to establish a cohort of well-defined CPHD patients.
Figure 1.
Flowchart on the formation of the patient cohort. Through a comprehensive search for panhypopituitarism and related ICD-9/10 diagnosis codes, we identified n=124 pediatric CPHD patients diagnosed or treated in the Helsinki University Hospital Children and Adolescents between 1985 and 2018. Patients with at least two pituitary hormone deficiencies were included in the study (Supplementary Table 2). Cases with partial laboratory testing were evaluated by experienced clinicians (PJM, MH, TR) based on patient records and growth charts.
ICD, International Classification of Diseases; CPHD, combined pituitary hormone deficiency; GH, growth hormone; GHD, growth hormone deficiency
The identified CPHD cases (n=196+23, Figure 1) were inspected to fulfill the inclusion criteria of at least two biochemically verified pituitary hormone deficiencies (for details, see Supplementary Table 2). Patient records and growth charts of patients with insufficient biochemical data were evaluated by experienced clinicians (PJM, MH, TR). The final study cohort comprised 124 patients (69 boys and 55 girls; Figure 1). Unequivocally subnormal hormone levels related to GH, TSH, and ACTH deficiency were present in 105, 78, and 29 patients, respectively. In others, hormone replacement therapy had been started either (i) based on clinical evaluation (e.g., after pituitary-hypophyseal region tumor resection), (ii) after partial laboratory testing of the pituitary function, or (iii) due to borderline low hormone level, and/or imminent progression of the deficiency in the context of high risk for hypopituitarism. Especially, the inclusion of gonadotropin deficiency and diabetes insipidus often required evaluation by the clinicians. In further analyses of the included patients, hormone replacement therapy is reported as a marker of pituitary hormone deficiency.
Clinical data
All diagnoses of the eligible patients were obtained from the patient records to classify acquired and congenital forms of CPHD. Acquired CPHD was defined to include CPHD of causes extrinsic to pituitary, and congenital CPHD included all idiopathic, syndromic, and genetic CPHD, regardless of the age at presentation. Presenting symptoms for all patients were collected. Age at presentation was the age at first visit or consultation by a HUH pediatric endocrinologist or pediatrician due to findings indicating the need for pituitary assessment. Age at diagnosis was the age at which the physician had recorded the deficiency of at least two pituitary hormones into the patient records. If not available, the date of commencement of hormonal treatment after the diagnosis of two pituitary hormone deficiencies was used.
Additional data collected for the patients with congenital CPHD included (i) birth data (pregnancy details, asphyxia and breech position at delivery, neonatal complications, and features suggesting hypopituitarism),36 (ii) phenotypic features, (iii) findings in brain MRI, (iv) family history of growth disorders and pituitary hormone deficiencies, and (v) auxological data (see next section). Septo-optic dysplasia (SOD) was defined as optic nerve hypoplasia (clinically or radiologically determined) in combination with hypopituitarism (self-evidently present in all our patients) and/or midline brain abnormalities.37 Regarding the neonatal phenotype of hypopituitarism, we categorized hypoglycaemia as a defined feature of CPHD, if the patient had had a hypoglycaemic seizure or apnea, or if she/he had been treated with intravenous glucose for >3 days, or at >3 days of age (persistent hypoglycaemia); other neonatal hypoglycaemia requiring any iv-glucose was defined as a non-specific cue. Similarly, we recorded jaundice as a defined feature, if phototherapy was given at >7 days of age, or with remark “prolonged jaundice”; otherwise, jaundice requiring any phototherapy was defined as a non-specific cue.36,38 We decided to include the slight, non-specific hypoglycaemia/jaundice phenotypes, since the duration or severity of the symptom had not been recorded for all patients, and, for some patients, the hormone replacement therapy was commenced so early that the persistence of the neonatal phenotype could not be assessed. However, we report the defined phenotypes separately. As for the neonatal genital phenotype, bilateral cryptorchidism and/or micropenis were accepted as signs of gonadotropin deficiency if stated in the patient records (information on penile length was not available for all patients). Preterm infants (born GW<37) were excluded from the neonatal phenotype analyses, since the probability of jaundice (both the severity and duration) and hypoglycemia among them are considerably higher than in full terms.39,40
We limited the incidence estimate of congenital CPHD to patients born between 2000 and 2018 in the HUH catchment area. The inclusion of patients from earlier years might have been more uncertain in terms of completeness of the patient search results and due to geographical changes in the catchment area. The number of these patients was compared to the number of live-born children in the Helsinki University Hospital catchment area between 2000 and 2018.41
Growth
Measurements of the patient's height, weight, and bone age were acquired from the electronic growth database (Pediator©; Tilator Oy). The auxological measurements had been carried out by trained nurses. Preterm infants (born GW<37) were excluded from the growth analyses, since catch-up growth in these patients is expected up to the age of 2,3 years, and reduced growth during the first years of life does not per se implicate the need for investigations of underlying pathology.42,43 To evaluate the growth deflection in patients with CPHD, we used the Finnish growth monitoring rules based on both static (deviation of height SDS (HSDS) from the patient's target height or the general population) and dynamic (deviation of HSDS from the patient's previous measurements) auxological criteria. These criteria are adjusted to detect the 0·5% most slowly and rapidly growing children in the general population for referral to further investigations.44 Isolated violation of the growth screening rules was, however, omitted, if subsequent growth was normal.
Genetics
Patients diagnosed with congenital CPHD lacking previous molecular genetic diagnoses were recruited to genetic studies. In total, 21 patients (16 directly recruited from the HUH registry, three based on Helsinki Biobank DNA samples in the HUH registry, and two from Kuopio University Hospital; only the 19 HUH patients were included in the clinical section of this paper), and 21 family members were enrolled. DNA samples from both parents were available for seven indexes, and from one parent for four indexes, while no family genetic data were available for ten indexes (Table 5).
Table 5.
Variants in the previously reported genes implicated in CPHD/GHD in patients with congenital CPHD.
| PatientID | Gender | Age at CPHD diagnosis (years) | Key phenotypic features | Genea | Variant description (nucleotide change; protein change; transcript; zygosity) | gnomAD highest population MAF (gnomAD MAF Finns) | rs-number | Classification according to ACMG/AMP 2015 guidelines | Number of family members/data available Variant carrying family members |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 4·0 | PSIS (GH, ACTH, TSH deficiencies). CC agenesis/hypoplasia, schizencephaly, frontomedial polymicrogyria, an arachnoid cyst. Global developmental disorder (Perisylvian syndrome) | ARNT2 | c.1707G>T; p.(Gln569His); ENST00000303329.9; het | 0·0048 (0·0018) |
rs145379118 | Uncertain significance | 2/0 NA |
| ·· | ·· | ·· | ·· | HMGA2 | c.307A>G; p.(Lys103Glu); ENST00000354636.7; het | 0·0078 (0·0078) |
rs77970919 | Likely benign | 2/0 NA |
| 2 | F | 0·2 | PSIS (GH, ACTH, TSH, gonadotropin deficiencies). Absent olfactory bulbs, Chiari I malformation. Bilateral cleft lip | CCDC88C | c.5087T>C; p.(Leu1696Pro); ENST00000389857.11; het | 0·0122 (0·0059) |
rs77154172 | Uncertain significance | 2/2 Healthy mother |
| ·· | ·· | ·· | ·· | CDON | c.3640G>A; p.(Gly1214Ser); ENST00000392693.7; het | 0·0018 (0·0018) |
rs189386496 | Likely benign | 2/2 Healthy mother |
| ·· | ·· | ·· | ·· | KIF14 | c.3808A>C; p.(Ser1270Arg); ENST00000367350.5; het | 0·0108 (0·0108) |
rs75449932 | Likely benign | 2/2 Healthy mother |
| ·· | ·· | ·· | ·· | MAGEL2 | c.137C>T; p.(Pro46Leu); ENST00000650528.1; het | 0·0013 (0·0013) |
rs1433820460 | Uncertain significance | 2/2 Healthy mother |
| ·· | ·· | ·· | ·· | RBM28 | c.2273A>G; p.(Asp758Gly); ENST00000223073.6; het | 0·0022 (0·0004) |
rs148028531 | Likely benign | 2/2 Healthy father |
| 3 | F | 0·7 | PSIS (GH, TSH deficiencies) | KIF14 | c.4013A>T; p.(Glu1338Val); ENST00000367350.5; het | 0·0144 (0·0053) |
rs77157287 | Likely benign | 2/1 Healthy mother |
| ·· | ·· | ·· | ·· | SHH | c.570G>A; p.(Ser190=); ENST00000297261.7; het | 0·0156 (0·00009549) |
rs9333633 | Uncertain significance | 2/1 - |
| ·· | ·· | ·· | ·· | WFS1 | c.1294C>G; p.(Leu432Val); ENST00000226760.5; het | 0·0138 (0) |
rs35031397 | Uncertain significance | 2/1 Healthy mother |
| 4 | M | 0·2 | GH, TSH, gonadotropin, ACTH deficiencies. EPP, optic nerve hypoplasia. Partial thalamus agenesis, dilated left lateral ventricle. Visual and hearing impairment | WFS1 | c.2611G>A; p.(Val871Met); ENST00000226760.5; het | 0·0127 (0·0127) |
rs71532874 | Likely benign | 2/0 NA |
| 5 | M | 5·1 | PSIS (GH, ACTH, TSH, gonadotropin deficiencies). Sloped shoulders, short neck. Small spleen and slightly small kidneys | CLCNKB | c.227G>A; p.(Arg76Gln); ENST00000375679.9; het | 0·0005 (0·0003) |
rs139676842 | Likely benign | 4/2 Healthy father |
| ·· | ·· | ·· | ·· | IGSF10 | c.4964C>T; p.(Ala1655Val); ENST00000282466.3; het | 0·0013 (0·0013) |
rs781205978 | Uncertain significance | 4/2 Healthy father |
| 6 | F | 4·8 | AP hypoplasia, EPP (GH, ACTH, TSH, gonadotropin deficiencies). Slightly short neck, slight craniofacial phenotype. Psychomotor developmental delay | ALMS1 | c.574G>A; p.(Glu192Lys); ENST00000613296.6; het | NA (NA) |
NA | Uncertain significance | 3/0 NA |
| ·· | ·· | ·· | ·· | CHD7 | c.8950C>T; p.(Leu2984Phe); ENST00000423902.7; het | 0·0132 (0·0132) |
rs184814820 | Likely benign | 3/0 NA |
| ·· | ·· | ·· | ·· | IFT172 | c.1459C>T; p.(Arg487Cys); ENST00000260570.8; het | 0·0081 (0·0081) |
rs143520040 | Uncertain significance | 3/0 NA |
| ·· | ·· | ·· | ·· | L1CAM | c.3508G>C; p.(Asp1170His); ENST00000370060.7; het | NA (NA) |
NA | Uncertain significance | 3/0 NA |
| ·· | ·· | ·· | ·· | WFS1 | c.2327A>T; p.(Glu776Val); ENST00000226760.5; het | 0·0129 (0·0129) |
rs56002719 | Uncertain significance | 3/0 NA |
| 7 | M | 4·7 | GH, TSH, gonadotropin deficiencies. MRI was not performed. | CHD7 | c.2503T>C; p.(Tyr835His); ENST00000423902.7; het | NA (NA) |
rs776581956 | Uncertain significance | 2/0 NA |
| ·· | ·· | ·· | ·· | FGFR1 | c.995G>A; p.(Gly332Asp); ENST00000425967.7; het | NA (NA) |
rs762320540 | Uncertain significance | 2/0 NA |
| ·· | ·· | ·· | ·· | HMGA2 | c.307A>G; p.(Lys103Glu); ENST00000354636.7; het | 0·0078 (0·0078) |
rs77970919 | Likely benign | 2/0 NA |
| KAT6A | c.5030_5031insACC; p.(Pro1677dup); ENST00000406337.6; het | 0·0034 (0·0003) |
rs758188280 | Uncertain significance | 2/0 NA |
||||
| ·· | ·· | ·· | ·· | SHH | c.251T>C; p.(Ile84Thr); ENST00000297261.7; het | NA (NA) |
NA | Uncertain significance | 2/0 NA |
| 8 | M | 14·6 | AP hypoplasia, EPP (GH, TSH deficiencies) | LAMB2 | c.4163G>A; p.(Arg1388Gln); ENST00000305544.9; het | 0·0026 (0·0026) |
rs146522641 | Likely benign | 3/2 Healthy sister |
| ·· | ·· | ·· | ·· | MAGEL2 | c.3017C>G; p.(Thr1006Ser); ENST00000650528.1; het | 0·006 (0·0047) |
rs138628273 | Likely benign | 3/2 - |
| 9 | F | 2·8 | Absent infundibulum, EPP (GH, TSH deficiencies). Bilateral optic nerve hypoplasia, CC agenesis, migration defect. Slight craniofacial phenotype. Visuomotor problems. Global developmental delay, epilepsy | GH1 | c.406G>A; p.(Val136Ile); ENST00000323322.10; het | 0·0091 (0·0025) |
rs5388 | Likely benign | 4/3 Healthy mother |
| ·· | ·· | ·· | ·· | IGSF10 | c.5620G>A; p.(Val1874Ile); ENST00000282466.3; het | 0·0193 (0·0193) |
rs145507750 | Likely benign | 4/3 Healthy mother |
| ·· | ·· | ·· | ·· | PITX2 | c.121C>T; p.(Pro41Ser); ENST00000644743.1; het | 0·0097 (0·0097) |
rs143452464 | Uncertain significance | 4/3 Healthy mother, healthy brother |
| ·· | ·· | ·· | ·· | PNPLA6 | c.4108G>A; p.(Gly1370Ser); ENST00000414982.7; het | 0·0086 (0·0086) |
rs145178162 | Likely benign | 4/3 Healthy mother |
| ·· | ·· | ·· | ·· | WFS1 | c.2611G>A; p.(Val871Met); ENST00000226760.5; het | 0·0127 (0·0127) |
rs71532874 | Likely benign | 4/3 Healthy father, healthy brother |
| 10 | M | 11·2 | Absent infundibulum, absent PP (GH, ACTH deficiencies; DI). Bilateral optic nerve hypoplasia, hypoplastic optic chiasm. Blindness, bilateral testis retention, developmental disability, epilepsy | ISL1 | c.1039A>G; p.(Ile347Val); ENST00000230658.12; het | 0·0001 (0) |
rs774986869 | Uncertain significance | 2/1 Healthy mother |
| 11 | M | 3·8 | PSIS (GH, TSH deficiencies). Slight craniofacial phenotype. Obesity, joint hypermobility. Speech and motor developmental disorder. | CHD7 | c.307T>A; p.(Ser103Thr); ENST00000423902.7; het | 0·0191 (0·0157) |
rs41272435 | Likely benign | 2/0 NA |
| ·· | ·· | ·· | ·· | PROP1 | c.425C>T; p.(Ala142Val); ENST00000308304.2; het | 0·0075 (0·0012) |
rs143790367 | Uncertain significance | 2/0 NA |
| 12 | F | 3·9 | PSIS (GH, ACTH, TSH deficiencies, likely HH) | CCDC88C | c.4265C>T; p.(Ser1422Leu); ENST00000389857.11; het | 0·0133 (0·0074) |
rs202217944 | Uncertain significance | 3/2 Healthy father |
| ·· | ·· | ·· | ·· | CHD7 | c.8950C>T; p.(Leu2984Phe); ENST00000423902.7; het | 0·0132 (0·0132) |
rs184814820 | Likely benign | 3/2 Healthy father |
| ·· | ·· | ·· | ·· | DCHS1 | c.7385G>A; p.(Arg2462Gln); ENST00000299441.5; het | 0·0194 (0·0072) |
rs117140835 | Uncertain significance | 3/2 Healthy mother |
| ·· | ·· | ·· | ·· | HNRNPU | c.2166_2167+1del; Splice site; ENST00000640218.2; het | 0·0053 (0) |
rs575582638 | Uncertain significance | 3/2 Healthy mother |
| ·· | ·· | ·· | ·· | ROBO2 | c.1793T>C; p.(Ile598Thr); ENST00000332191.12; het | 0·0129 (0·0004) |
rs185792666 | Likely benign | 3/2 Healthy mother |
| ·· | ·· | ·· | ·· | SHH | c.676G>A; p.(Ala226Thr); ENST00000297261.7; het | 0·0001 (0) |
rs104894043 | Likely pathogenic | 3/2 Healthy mother |
| 13 | F | 1·1 | PSIS (GH, TSH, gonadotropin deficiencies; mild ACTH deficiency). Unilateral visual defect, midfacial hypoplasia, slightly short neck, arthrogryposis, swallowing problems, GI motility problems. | IGSF10 | c.4607T>C; p.(Ile1536Thr); ENST00000282466.3; het | 0·0181 (0·0181) |
rs138084379 | Likely benign | 2/0 NA |
| ·· | ·· | ·· | ·· | RBM28 | c.746A>G; p.(Asp249Gly); ENST00000223073.6; het | 0·0084 (0·0084) |
rs145277422 | Likely benign | 2/0 NA |
| 14 | M | 0·0 | PSIS (GH, ACTH, TSH, gonadotropin deficiencies). Probable olfactory bulb hypoplasia, obstructive hydrocephalus; multiple other brain malformations including midbrain, hippocampal, pons, cerebellar vermis and Sylvian fissure anomalies. Congenital bilateral ptosis, anisocoria, papillary defect (colobomas), visuomotor problems. Micropenis, cryptorchidism, anterior anus. von Willebrand's disease | TBC1D32 | c.1166_1167insGT; p.(Gln390PhefsTer32); ENST00000275159.10; het | 0·0099 (0·0099) |
rs546631812 | Uncertain significance | 3/1 - |
| 15 | M | 1·1 | PSIS (GH, ACTH, TSH deficiencies). Slightly thin CC. Bilateral optic nerve hypoplasia. Delayed language development. | GLI3 | c.3664C>T; p.(Pro1222Ser); ENST00000395925.8; het | 0·006 (0·0011) |
rs118149040 | benign | 5/3 Healthy father |
| 16 | F | 0·1 | Thin infundibulum, EPP (GH, TSH deficiencies; transient ACTH deficiency). Curved CC, absent septum pellucidum, incomplete hippocampal rotation. Prominent forehead | LHX3 | c.934C>G; p.(Arg312Gly); ENST00000371746.9; het | 0·0002 (0·0002) |
rs145867977 | Likely benign | 2/2 Healthy father |
| 17 | M | 0·0 | Absent infundibulum, partial EPP (GH, ACTH, FSH/LH deficiencies; possible TSH deficiency). Optic nerve hypoplasia, visual defect and unilateral blindness. Bilateral testis retention. Slight craniofacial phenotype | DCHS1 | c.5503C>T; p.(Leu1835Phe); ENST00000299441.5; het | 0·0022 (0·0022) |
rs148791938 | Uncertain significance | 2/0 NA |
| ·· | ·· | ·· | ·· | IGSF10 | c.4607T>C; p.(Ile1536Thr); ENST00000282466.3; het | 0·0181 (0·0181) |
rs138084379 | Likely benign | 2/0 NA |
| 18 | F | 0·0 | PSIS (GH, ACTH, TSH deficiencies). Optic nerve hypoplasia, hypoplastic papillae, visual defect and unilateral blindness. | IGSF10 | c.1720G>A; p.(Glu574Lys); ENST00000282466.3; het | 0·0168 (0·0168) |
rs116716539 | Uncertain significance | 2/2 Healthy mother |
Previously published genes implicated in CPHD/GHD are shown in Supplementary Table 1.
CPHD, combined pituitary hormone deficiency; MAF, minor allele frequency; ACMG; American College of Medical Genetics; AMP, the Association for Molecular Pathology; GHD, growth hormone deficiency; PSIS, Pituitary stalk interruption syndrome; GH, growth hormone; ACTH, adrenocorticotropin; TSH, thyrotropin; NA, not applicable; CC, corpus callosum; EPP, ectopic posterior pituitary; AP, anterior pituitary; DI, diabetes insipidus; HH, hypogonadotropic hypogonadism; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Whole exome sequencing was performed at the sequencing laboratory of the Institute for Molecular Medicine Finland (FIMM) Technology Centre, University of Helsinki. The DNA of the study subjects was extracted from peripheral blood leukocytes and the whole exome sequencing was performed with Illumina Novaseq SP PE150, Novaseq S1 PE150, or Novaseq S1 PE101 technology. First, the adapter was trimmed from the reads, as well as any low-quality nucleotides from the 5’ or 3’ ends of the read, removing pairs with less than 36 bp. The reads were aligned to the GRCh38 reference genome with the BWA (Burrows-Wheeler Aligner). Non-unique read pairs and non-unique single reads were removed and GATK Base Recalibrator was used to clean the alignment. Any potential PCR duplicates were removed using Picard MarkDuplicates, and GATK IndelRealigner was used for indel sites. The mpileup from the SAMTOOLS package was used for variant calling.45 The sequencing yielded 90% 20x coverage.
The subjects’ VCP files were annotated using ANNOVAR.46 For a variant to be potentially causative and thus eligible for further analysis, we determined that the variant (i) should be in the previously published genes implicated in CPHD/GHD (n=80, Supplementary Table 1), (ii) be either exonic and nonsynonymous, or lie in the consensus splice site or its proximity, which we defined as within ten bases from the splice site, (iii) have a reported minor allele frequency (MAF) equal to or below 2% in all subpopulations in the gnomAD (provided in the ANNOVAR annotations), dbSNP (https://www.ncbi.nlm.nih.gov/snp/), and SISu (http://www.sisuproject.fi/) databases (manually verified).47,48 Variants fulfilling these criteria were classified according to the ACMG/AMP 2015 guidelines using the InterVar bioinformatics software tool.49,50
Ethics
The patient studies (direct patient recruitment and the Biobank study) had been approved by the Ethics Committee of Helsinki University Hospital. Genetic studies were carried out according to the Declaration of Helsinki. All directly recruited patients and their guardians gave their written informed consents to participate in the study.
Statistical analyses
The data were analyzed using Microsoft Excel 2016, SPSS statistical software, (SPSS, Version 25.0. Armonk, NY: IBM Corp), and R version 4.0.3. To assess the variable distribution, we visualized the distribution and assessed skewness and kurtosis parameters. Additionally, normality was tested with Shapiro-Wilk test of normality, acknowledging that for small samples, the statistical power of the test may not be sufficient to reject the null hypothesis. Mann-Whitney U-test was used to evaluate the statistical differences in the ages at presentation and diagnosis between the patients with acquired and congenital disease. For auxological data, Mann-Whitney U-test was used to compare Groups I and II for target height, growth screening measures, delay to GH treatment, and latest height measures. T-test was used for birth measures. Wilson score interval was used to estimate the population-level incidence of congenital CPHD. Weighting was not considered necessary for the estimate, due to the representativeness of the patient cohort in both national and international context, and the lack of previous incidence estimates in a similar patient population (for further details, please see Discussion). The data are reported with mean±SD or median interquartile range (IQR) based on the variable distribution.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to study data and approved the manuscript for publication.
Results (V体育ios版)
Overview of the etiology of CPHD
We investigated the etiologies and diagnostic features of CPHD in the largest tertiary center in Finland. The etiologic spectrum is presented in Table 1. The majority (61%;76/124) of the cases had acquired CPHD, with brain tumors (mainly craniopharyngiomas and gliomas) as the most common etiology. Rare acquired etiologies included iatrogenic, brain trauma, and inflammatory/infiltrative diseases. Also, as we aimed to differentiate developmental mechanisms of hypopituitarism from external causes, one patient with empty sella and hypopituitarism conceivably due to cerebral fluid compression of the pituitary, was classified as acquired CPHD. Congenital CPHD was found in 48 (39%) patients, of whom seven (15%) had a defined genetic diagnosis. One patient with congenital CPHD had died during early childhood despite adequate hormone substitution treatment.23
Table 1.
The causes of panhypopituitarism in pediatric patients diagnosed or treated at Helsinki University Hospital between 1985 and 2018.
| n (%) boys | n (%) girls | n (%) total | |
|---|---|---|---|
| Acquired | 41 (59) | 35 (64) | 76 (61) |
| Brain tumor and its treatment | 34 (49) | 33 (60) | 67 (54) |
| Craniopharyngioma | 15 (22) | 14 (25) | 29 (23) |
| Gliomas | 8 (12) | 6 (11) | 14 (11) |
| Pilocytic astrocytoma | 5 (7) | 4 (7) | 9 (7) |
| Glioma as part of neurofibromatosis I | 1 (1) | 2 (4) | 3 (2) |
| Glioma, other | 2 (3) | 2 (4) | 4 (3) |
| Glioma, not classified | 1 (1) | 0 (0) | 1 (1) |
| Germinal tumor | 2 (3) | 6 (11) | 8 (6) |
| Pituitary adenoma | 2 (3) | 1(2) | 3 (2) |
| Other | 6 (9) | 4 (7) | 10 (8) |
| Iatrogenic | 1 (1) | 1 (2) | 2 (2) |
| Hemispherectomy due to epilepsy | 1 (1) | 0 (0) | 1 (1) |
| Treatment of beta thalassemia | 0 (0) | 1 (2) | 1 (1) |
| Other | 6 (9) | 1 (2) | 7 (6) |
| Brain trauma | 1 (1) | 0 (0) | 1 (1) |
| Empty sellaa | 1 (1) | 0 (0) | 1 (1) |
| Histiocytosis X/Langerhans cell histiocytosis | 3 (4) | 0 (0) | 3 (2) |
| Hypophysitis (autoimmune) | 1 (1) | 0 (0) | 1 (1) |
| Infundibular epidermoid cyst | 0 (0) | 1 (2) | 1 (1) |
| Congenital | 28 (41) | 20 (36) | 48 (39) |
| Idiopathic | 17 (25) | 12 (22) | 29 (23) |
| Abnormal pituitary MRI | 11 (16) | 11 (20) | 22 (18) |
| Normal pituitary MRI | 1 (1) | 0 (0) | 1 (1) |
| No MRI investigation | 5 (7) | 1 (2) | 6 (5) |
| Genetically verified | 4 (6) | 3 (5) | 7 (6) |
| PROP1 | 1 (1) | 1 (2) | 2 (2) |
| SOX3 | 1 (1) | 0 (0) | 1 (1) |
| SOX2 (Septo-optic dysplasia) | 0 (0) | 1 (2) | 1 (1) |
| OTX2 | 1 (1) | 0 (0) | 1 (1) |
| TBC1D32 | 1 (1) | 1 (2) | 2 (2) |
| Known syndromes without genetic diagnosis | 7 (10) | 5 (9) | 12 (10) |
| Septo-optic dysplasia | 7 (10) | 5 (9) | 12 (10) |
Empty sella is listed among acquired causes of panhypopituitarism, since congenital hypopituitarism was defined to include idiopathic, genetic, and syndromic hypopituitarism (suspected developmental etiologies of the hypothalamic-pituitary axis) only.
According to our inclusion criteria, a retrospectively verifiable biochemical or clinical GH, TSH, or ACTH deficiency, or diabetes insipidus (DI) was present in 120, 109, 69, and 23 of the 124 patients, respectively. However, 60 patients in total received desmopressin treatment for DI since this had often been started based on the treating clinician's interpretation and partial laboratory findings suggesting DI. Sex steroid replacement therapy had been commenced in 52% of patients (n=64, of whom 34 females) at the mean age of 14·1±1·9 years.
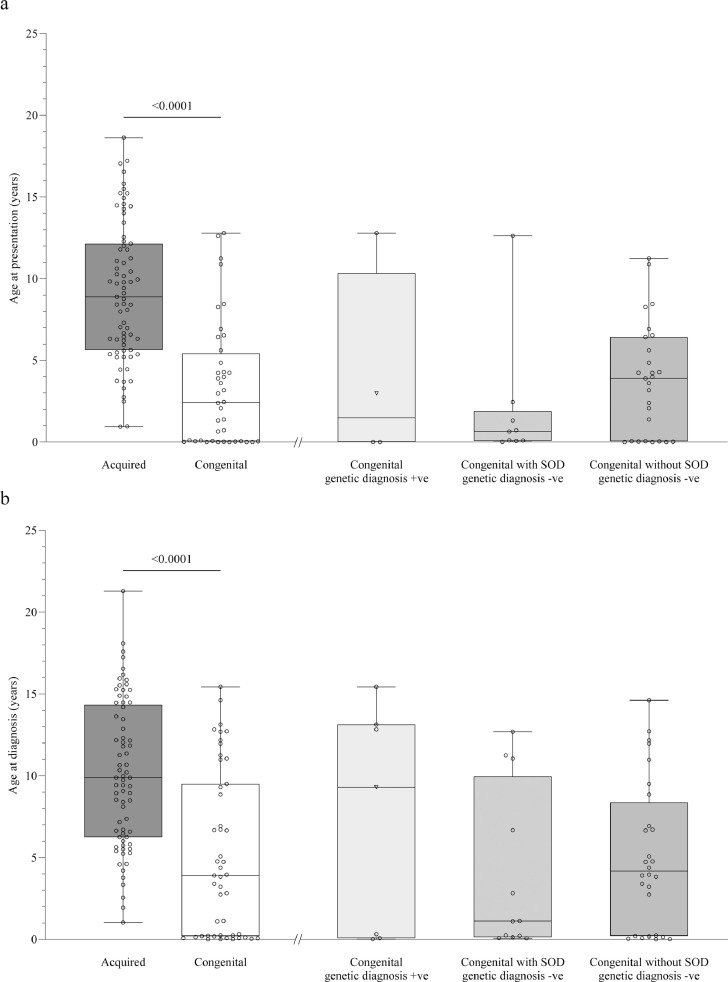
The age at presentation and diagnosis for acquired and congenital CPHD are shown in Figure 2. Patients with congenital CPHD presented at a younger age (median 2·4, IQR 0·5 to 5·4 years) compared to patients with acquired CPHD (median 8·9, IQR 5·6 to 12·1 years) p<0·0001. Similarly, the age at diagnosis was lower in patients with congenital (median 3·9, IQR 0·2 to 9·5 years) than acquired (median 9·9, IQR 6·2 to 14·3 years) CPHD p<0·0001.
Figure 2.
The etiology of CPHD varies according to the age at presentation and diagnosis. Scattered min-max boxplots depicting the age at presentation (panel a) and age at diagnosis (panel b) for patients with acquired and congenital CPHD. For patients with congenital CPHD, the data is additionally shown as divided into main diagnostic subgroups. For one patient with congenital CPHD and an established genetic diagnosis, the phenotype was consistent with SOD. The patient is depicted as a triangle in the ‘Congenital with genetic diagnosis +ve’ group.
Panel a: Patients with congenital CPHD presented at a younger age compared to acquired CPHD, p<0·0001, effect size r=-0·573. Data were available in 71/76 and 40/48 patients with acquired and congenital CPHD, respectively.
Panel b: Patients with congenital CPHD received the diagnosis at a younger age compared to acquired CPHD p<0·0001, effect size r=-0·451. Data were available in 73/76 and 47/48 patients with acquired and congenital CPHD, respectively.
+ve, positive; -ve, negative; SOD, septo-optic dysplasia; CPHD, combined pituitary hormone deficiency.
Phenotypic features of acquired CPHD
Patients with acquired CPHD often presented to medical investigations due to neurologic symptoms of the underlying disease, evident in 54 (71%) patients. These included ophthalmic manifestations (e.g., poor vision), symptoms related to increased intracranial pressure (nausea and headache), deterioration in motor skills, and non-specific symptoms, such as tremor, fatigue, and memory problems. Symptoms related to hypopituitarism (reduced growth, delayed puberty, and/or signs of DI) were among the presenting symptoms in 23 (30%) patients, of whom seven also had neurologic or non-specific symptoms. Of the 67 patients with tumor etiology, CPHD was diagnosed in 19 at presentation and in 32 after brain surgery (often shortly after the first presentation due to tumor-related symptoms but in a few patients after reoperation). In 15 tumor patients, CPHD occurred during follow-up after tumor treatment (including chemotherapy/irradiation). For one patient, the data was not available.
Phenotypic features of congenital CPHD
The deficiency of all anterior pituitary hormones (GH, ACTH, TSH, and FSH/LH) was present in 14 (30%) patients with congenital CPHD (Supplementary Table 3). Other frequent combinations of hormone deficiencies included GH and TSH (n=9, 19%), GH, TSH, and FSH/LH (n=8, 17%), and GH, TSH, and ACTH (n=6, 13%). DI was present in seven (15%) patients. IGHD had preceded the development of CPHD in six (14%) patients (data available in 43/48).
Of the 48 patients with congenital CPHD, eight (17%) were born in breech position, and four (8%) had suffered from birth asphyxia. Neonatal signs associated with hypopituitarism were present in 26 (74%) patients (Figure 3). Six patients were excluded from the neonatal phenotype analysis due to prematurity and seven due to lack of data. A defined neonatal phenotype (see Materials and Methods) was present in 23 (66%) patients. Of these patients, 68% had received a CPHD diagnosis during the first six months of their life (data available in 22/23), including all eight male patients with a genital phenotype. Five patients with defined neonatal signs of hypopituitarism (24%; data available in 21/23), who had either isolated hypoglycaemia or isolated jaundice, did not present to endocrine investigations during the first year of their life.
Figure 3.
Neonatal features suggesting hypopituitarism in patients with congenital CPHD. Neonatal features were present in 26/35 patients; 23 patients had neonatal hypoglycaemiaa, 17 patients had neonatal jaundiceb, and eight male patients presented with a neonatal genitalc phenotype. The quantitative Venn diagram indicates the approximate proportional quantities and overlap of the respective features.
a any note of neonatal hypoglycaemia and intravenous glucose treatment in the patient charts; 16/23 patients with hypoglycaemia had defined hypoglycaemia (a hypoglycaemic seizure or apnea, or treatment with intravenous glucose for >3 days, or at >3 days of age).
b patients who had received phototherapy, or had the remark “prolonged jaundice” in the patient charts; 9/17 of the patients with jaundice had defined jaundice (phototherapy at >7 days of age, or remark “prolonged jaundice”)
c micropenis and/or bilateral cryptorchidism.
CPHD, combined pituitary hormone deficiency.
Of the eight patients with neonatal micropenis/cryptorchidism, six had biochemical evidence of absent minipuberty (tested between the age of 2 and 14 weeks), seven received treatment during minipuberty (five patients with recombinant FSH+testosterone51 and two with testosterone only). Two patients had been followed up until the time of puberty and required testosterone supplementation, and four patients were still of prepubertal age. All these patients also had GHD as part of their CPHD.
Brain MRI was investigated in 41 (85%) patients with congenital CPHD (Table 2). All of them had abnormalities in the brain MRI. Classic pituitary abnormalities associated with CPHD (anterior pituitary hypoplasia, absent or ectopic posterior pituitary, infundibulum hypoplasia, or the combination of these (pituitary stalk interruption syndrome, PSIS)) were identified in 37 (90%) patients. Extra-pituitary brain anomalies were present in 25 (61%) patients.
Table 2.
Brain MRI findings in patients with congenital CPHD.
| n (%) patients | |
|---|---|
| Classic pituitary findings associated with CPHD | 37 (90) |
| Pituitary stalk interruption syndrome | 21 (51) |
| Anterior pituitary hypoplasia, other | 9 (22) |
| Absent/ectopic posterior pituitary, other | 11 (27) |
| Hypoplastic infundibulum, other | 7 (17) |
| Other MRI findings | 25 (61) |
| Thick hypophysis | 1 (2) |
| Thick and then regressing infundibulum | 1 (2) |
| Small hypothalamus | 1 (2) |
| Optic nerve hypoplasia/atrophy | 8 (20) |
| Corpus callosum hypoplasia/agenesis | 6 (15) |
| Absent/anomal septum pellucidum | 6 (15) |
| Olfactory bulb hypoplasia | 3 (7) |
| Polymicrogyria | 3 (7) |
| Schizencephaly | 1 (2) |
| Chiari I | 1 (2) |
| Communicating hydrocephalus | 1 (2) |
| Obstructive hydrocephalus | 1 (2) |
| Other structural defects of the braina | 11 (24) |
Brain MRI was investigated in 41/48 of the patients.
Finding (n patients): arachnoid cyst (3), local heterotopy (3), other local cortex structural anomaly (2), thalamus hypoplasia/agenesis (2), partial fornix dislocation/anomaly (2), slight pons anomaly (2), potential hemosiderin collection (1), frontal cavernoma (1), small cyst (1), interhypothalamic adhesion (1), cerebral peduncle and mamillary body anomalies (1), hippocampal anomaly (1), cerebellar vermis anomaly (1), Sylvian fissure anomaly (1), wide foramen magnum (1), dilated left lateral ventricle (1).
CPHD, combined pituitary hormone deficiency.
Of the patients with neonatal features of CPHD, 22 (85%) had brain MRI investigated. PSIS was present in 14 (64%) and various other combinations of anterior pituitary hypoplasia, infundibulum hypoplasia, and absent/ectopic posterior pituitary in seven (32%) of them, including isolated ectopic posterior pituitary in two. Extra-pituitary MRI findings, present in 15 (68%) included SOD features in 11. Optic nerve hypoplasia was bilateral in 2/5 cases. Four patients (of whom three with SOD) had schizencephaly, local polymicrogyria, local cortical structural abnormality, and/or heterotopic nodules that have previously been associated with, e.g., SOD.52 Olfactory bulb hypoplasia was present in three.
Extrapituitary phenotypes were found in 35 (73%) congenital CPHD patients: craniofacial (n=13, 27%), eye (n=16, 33%), genital (n=10, 21%), and developmental delay/epilepsy (n=18, 38%) phenotypes as the most frequent findings (Table 3). Features consistent with SOD were detected in 13 patients with CPHD (Table 1). Their age at CPHD diagnosis was highly variable from early infancy (n=6) to 11-12 years of age (n=3). Eight out of the 12 SOD patients with available patient data had presented with neonatal features of hypopituitarism, and six of them had received a neonatal CPHD diagnosis.
Table 3.
The extrapituitary phenotypic findings in the 48 patients with congenital CPHD.
| n (%) patients | |
|---|---|
| Patients with extrapituitary phenotypes | 35 (73) |
| Craniofacial | 13 (27) |
| Defined | 5 (10) |
| Mild features reported in patient charts | 8 (17) |
| Eye | 16 (33) |
| Genital | 10 (21) |
| Ear | 2 (4) |
| Musculoskeletal | 4 (8) |
| Arthrogryposis | 1 (2) |
| Short neck (clinical evaluation from patient charts) | 3 (6) |
| Developmental delay/epilepsy | 18 (38) |
| Dental | 4 (8) |
| Thermoregulation abnormality | 1 (2) |
CPHD, combined pituitary hormone deficiency.
Growth of patients with congenital CPHD
Height growth was analyzed in 26 (54%) patients with congenital CPHD; other patients were excluded due to prematurity (n=6), missing data (n=10), and start of GH Rx treatment without preceding abnormal height growth according to the Finnish growth screening rules (n=6). Height growth deflection was evident in 12 (46%) patients in early infancy (0–6 months of age; Group I) and in 11 (42%) patients between six months to four years of age (Group II). Key auxological measurements for Group I and II are presented in Table 4.
Table 4.
Key auxological measurements for patients with congenital CPHD.
| Group Ia | Group IIb | p-value (effect size) | |
|---|---|---|---|
| Number of patientsc | 12 | 11 | ·· |
| Birth data | ·· | ·· | ·· |
| data availability: n | 12 | 11 | ·· |
| Birth length (SDS): mean (SD) | -0·4 (1·2) | 0·2 (0·9) | p=0·117 (d=-0·513)d |
| Birth weight for height (%): mean (SD) | 5 (11) | 0 (8) | p=0·113 (d=0·521)d |
| Target height (TH) | ·· | ·· | ·· |
| data availability: n | 10 | 10 | ·· |
| TH SDS: median (IQR) | -0·2 (-0·4 to 1·1) | -0·3 (-0·5 to 0·2) | p=0·353 (r=0·221)e |
| TH SDS: range | -0·6 to 1·4 | -0·7 to 0·4 | ·· |
| First violation of the growth screening rules | ·· | ·· | ·· |
| data availability: n | 8 | 11 | ·· |
| HSDS: median (IQR) | -3·1 (-3·9 to -1·5) | -2·5 (-2·8 to -1·8) | p=0·272 (r=0·266)e |
| HSDS: range | -4·7 to -1·1 | -3·0 to -1·3 | ·· |
| Age (years): median (IQR) | 0·3 (0·3 to 0·5) | 1·8 (1·3 to 2·0) | p<0·0001 (r=0·845)e |
| Age (years): range | 0·3 to 0·5 | 0·8 to 4·0 | ·· |
| Delay to GH treatment | ·· | ·· | ·· |
| data availability: n | 8 | 11 | ·· |
| years: median (IQR) | 1·9 (0·6 to 6·1) | 2·5 (1·2 to 3·2) | p=0·778 (r=-0·076)e |
| years: range | 0·0 to 11·0 | 0·6 to 7·0 | ·· |
| Latest height measurement | ·· | ·· | ·· |
| data availability: n | 11 | 11 | ·· |
| HSDS: median (IQR) | -0·6 (-2·1 to 0·1) | -0·6 (-1·6 to 0·0) | p=0·797 (r=0·063)e |
| HSDS: range | -4·8 to 0·8 | -2·7 to 0·8 | ·· |
| Age (years): median (IQR) | 16·7 (8·5 to 17·7) | 15·2 (13·6 to 18·2) | p=0·847 (r=0·042)e |
| Age (years): range | 2·9 to 19·0 | 9·9 to 19·7 | ·· |
Group I, patients with HSDS deviation during the first six months of life; four patients with the start of GH Rx treatment prior to the age of 0·25 years included only in the birth and final height measures.
Group II, patients with HSDS deviation between six months to four years of age.
Data available for 26 patients. Two patients who had not been diagnosed with GHD and one with growth deviation first at the age of 12 years are not shown.
T-test and Cohen's d.
Mann-Whitney U-test and r.
CPHD, combined pituitary hormone deficiency; HSDS, height SDS; GHD, growth hormone deficiency.
Birth HSDS, birth weight for height, and target height of patients with congenital CPHD were close to the general population (Table 4). Defined neonatal features of hypopituitarism were present in 8/12 patients of Group I and in 5/11 patients of Group II. Further, these were among the presenting symptoms to endocrine investigations in 7/7 patients of Group I and 1/4 patients of Group II, for whom the data were available (data not shown). This suggests that the patients with earlier growth retardation (Group I) had a more severe CPHD phenotype in early infancy. Also, none of the patients in Group II had presented with a neonatal genital phenotype. Reduced height growth was the presenting symptom in endocrine investigations of 11/23 (42%) congenital CPHD patients; 3/12 (25%) in Group I and 8/11 (73%; the only presenting symptom in 45%) in Group II.
The median delay between the first violation of the growth screening rules and the initiation of GH Rx treatment among all congenital CPHD patients was 2·2 years (IQR 1·2 to 3·7 years; n=19). There was no statistical difference in the median delay to GH Rx between Group I (1·9, IQR 0·6 to 6·1 years) and Group II (2·5, IQR 1·2 to 3·2 years), p=0·778. Figure 4 presents the height growth of patients in Group I and Group II. To more reliably estimate the GHD diagnostic delay, we analyzed the delay between the violation of the growth screening rules and the presentation at pediatric endocrinologist for pituitary assessment, in the subgroup of patients referred to investigations due to divergent growth. This delay was median 2·2 years (IQR 1·2 to 3·6 years, n=11). Only two of these patients presented for evaluation of the pituitary function during the year following the growth screening alert.
Figure 4.
Individual growth curves of patients with congenital CPHD including GHD prior to treatment with GH Rx, or until 6 years of age, if no previous GH supplementation.
Panel a: Patients with growth deflection at the age of ≤6 months (Group I, n=8; four patients with the start of GH Rx treatment prior to the age of 0·25 years not depicted); Panel b: Patients with growth deflection at the age of 6 months to 4 years (Group II, n=11). One patient with the first growth deflection at the age of 12 years not shown.
HSDS, Height SDS.
Incidence estimate for congenital CPHD
During the years 2000-2018, 422 258 live children were born in the Helsinki University Hospital catchment area41 and 26 patients born in this region during the same period were diagnosed with congenital CPHD, giving an estimated incidence of 26/422 258 (1 in 16 000 live-born children, 95%CI 1/11 000-1/24 000) for congenital CPHD.
Genetics
In previous clinical or research investigations, a molecular genetic cause of CPHD had been identified in seven patients: a homozygous deletion of PROP1 in two siblings of Kurdish origin with CPHD, compound heterozygous loss-of-function variants in TBC1D32 in siblings with PSIS, and mild craniofacial dysmorphism; a previously described loss-of-function variant in SOX2 in a female patient with SOD (anophthalmia and CPHD), a Xq27.1 microduplication of unknown size leading to loss of SOX3 in a male patient with PSIS, and a frameshift variant in OTX2 in a male patient with PSIS and retinitis pigmentosa.23,53, 54, 55
Of the 21 patients who were enrolled in genetic investigations, 18 carried rare (MAF≤2%) nonsynonymous or consensus splice site variants in genes implicated in CPHD/GHD, as shown in Table 5. Due to incomplete sequence data availability from family members, we were unable to analyze the mode of inheritance for most variants. Variants with MAF either ≤0·5% or not reported, were identified in ten indexes in 16 different genes (ALMS1, ARNT2, CDON, CHD7, CLCNKB, DCHS1, FGFR1, IGSF10, ISL1, KAT6A, L1CAM, LAMB2, LHX3, MAGEL2, RBM28, and SHH). However, according to the ACMG/AMP 2015 guidelines, classifications for most variants were “Uncertain significance” or “likely benign”. The rare variants identified in our study were all heterozygous, and, considering the incidence estimate of CPHD presented above, the possibility of an autosomal dominant monogenic disease could only be considered in patients carrying extremely rare variants. Limiting to variants with MAF≤0·01% or not reported and excluding those classified as “benign” or “likely benign”, we encountered variants in ISL1 (patient #10); SHH (patient #12); ALMS1 and L1CAM (patient #6); and FGFR1, CHD7, and SHH (patient #7). The only variant classified as “likely pathogenic'', was the heterozygous SHH c.676G>A, p.(Ala226Thr) variant carried by patient #12 with PSIS (GH, ACTH, TSH deficiencies, and estrogen treatment due to suspected partial gonadotropin deficiency) and her unaffected mother.
"V体育2025版" Discussion
Little is known about the incidence of congenital CPHD. The available incidence estimates range from 1/4000-1/10 000, but in fact, these are based on variable short stature and growth hormone deficiency incidence/prevalence estimates and on one optic nerve hypoplasia study.56, 57, 58, 59, 60, 61 We studied pediatric CPHD patients treated in the largest tertiary center in Finland, and, after inclusion of only patients with two or more pituitary hormone deficiencies, were able to provide an incidence estimate for congenital CPHD of 1 in 16 000 children.
Persistent neonatal hypoglycemia, jaundice, and genital hypoplasia are important cues to early diagnosis of congenital CPHD.36 In 66% of our patients, a defined neonatal hypopituitarism phenotype was present. Almost one-third of the boys with congenital CPHD had micropenis and/or bilateral cryptorchidism. Half of all congenital CPHD patients had received phototherapy or exhibited prolonged jaundice, and 66% had required neonatal iv-glucose infusion or exhibited a hypoglycaemic seizure during the first weeks of life. These frequency estimates are clearly higher than those among healthy neonates and infants (cryptorchidism: 1 to 9% of full-term boys; phototherapy: up to 8% of term/late preterm infants; occurrence of low plasma glucose, usually noted as <2.2 to 2.5 mmol/L: 5% to 15% of normal newborn infants),62, 63, 64 yet the symptoms per se are highly unspecific and therefore easily missed as a sign of a rare disease such as pituitary hormone deficiency.65,66 As suggested before, prolonged and severe neonatal hypoglycemia and jaundice should trigger the suspicion of CPHD.38,67 In contrast to the results by Jullien et al., neonatal genital phenotype was in our series a cue to early diagnosis of CPHD.17 Also, the boys with a neonatal genital phenotype developed GHD and most of them could be verified with gonadotropin deficiency. In our series, patients with combinations of defined neonatal features of CPHD were identified early. However, isolated hypoglycaemia or jaundice did not always trigger endocrine investigations, and a substantial proportion (24%) of the patients with defined neonatal features suggesting hypopituitarism presented to endocrine investigations only after their first birthday.
The majority of the congenital CPHD patients had classic findings of severe hypopituitarism in the brain MRI: anterior pituitary hypoplasia, absent or ectopic posterior pituitary, infundibulum hypoplasia, or the combination of these (PSIS). Over half of the patients also had extra-pituitary defects in the MRI. Especially craniofacial, eye, and developmental delay/epilepsy phenotypes were common in congenital CPHD patients; these features were present in approximately one-third of the patients. The pituitary does share multiple developmental factors with the eye and brain midline structures. Developmental delay phenotypes have been linked to, e.g., BMP4 and LHX3 variants, and CPHD-associated syndromes, such as SOD and CHARGE.61,68
We analyzed the growth of congenital CPHD patients using the Finnish growth screening rules.44 These rules detect the 0·5% of most divergently growing children from the general population for further evaluation in tertiary healthcare. Height growth retardation was evident in half of the growth hormone deficient children already during the first six months of life, but in the others mostly between the age of six months to four years. This is in line with Wit et van Unen (1992), who described 15 CPHD patients, 50% of whom presented with early reduced growth.36 However, Mehta et al. described 44 GHD and CPHD patients, all of whom exhibited a decrease of HSDS within the first six months of life.26 In our study, 8/14 males included in the growth analysis presented with early growth retardation during the first six months of life, including all six patients with a neonatal genital phenotype suggestive of congenital gonadotropin deficiency. While neonatal phenotypic features of CPHD led to investigations of CPHD in many patients with early growth retardation, 25% of them presented due to reduced growth only, which highlights the importance of growth monitoring in the early identification of CPHD.
The median delay from the first violation of the growth screening rules to GH treatment among those with congenital CPHD was remarkably long (median 2·2, IQR 1·2 to 3·7 years). This applied also to the subgroup of patients who presented to endocrine investigations due to reduced growth (median delay to presentation to CPHD investigations 2·2 years (IQR 1·2 to 3·6 years). This is in agreement with the results by Gascoin–Lachambre et al, who reported a 2·3-year median diagnostic delay in 21 patients with GHD or CPHD and PSIS.69 In our series, explanations for the delay to GH treatment were watchful waiting during early years of life, if no symptomatic hypoglycaemia or other complications occurred, and the investigations and treatment of more common causes of growth retardation, such as hypothyroidism and infections (data not shown). In isolated patients, other conditions, such as sleep apnea or exogenous cortisone treatment of asthma appeared the plausible explanation of reduced growth and delayed the GHD investigations. However, in some patients, the early diagnostic opportunity of CPHD based on growth retardation was indeed missed.
Among the HUH patients with congenital CPHD, seven (15%) had a previous molecular genetic diagnosis, including one patient with SOD. In our molecular genetic investigations of 21 patients with congenital CPHD, no new conclusive molecular genetic diagnoses (that is, a pathogenic variant according to the ACMG/AMP 2015 guidelines in genes implicated in CPHD/GHD) were made. One variant in SHH, c.676G>A, p.(Ala226Thr), carried by patient #12 with PSIS and her healthy mother, was classified as “likely pathogenic”, and thus the variant is the probable cause for the patient's disease. The variant has previously been identified in a Dutch patient with PSIS and CPHD, and in a familial case of holoprosencephaly.70,71 In our patient and the two previous cases, the variant was also present in clinically unaffected parents suggesting incomplete penetrance, a phenomenon often described in both familial CPHD and holoprosencephaly. (e.g.,14,20,70,72, 73, 74) In addition to the variant in SHH, patient #12 carried rare variants in five other genes implicated in CPHD/GHD. Similarly, patients #2, #6, #7, and #9 carried rare variants in five genes implicated in CPHD/GHD. Although these variants were inherited from healthy family members or their segregation could not be assessed, we cannot rule out the possibility that the variants (especially those of “unknown significance”) could have contributed to CPHD. As to the extremely rare (MAF≤0·01% or not reported) variants classified as “uncertain significance” (ISL1 (patient #10); ALMS1 and L1CAM (patient #6); and FGFR1, CHD7, and SHH (patient #7)), the phenotype of patient #6 did not match the phenotypes or the mode of inheritance related to ALMS1 or L1CAM in previous literature (Table 5 and Supplementary Table 4), whereas the phenotypic comparison did not further support or exclude the role of the identified variants in patients #7 and #10. Taken together, the 15% frequency of conclusive genetic diagnoses in congenital CPHD is in line with the previous frequency estimate (12·4%) for pathogenic variants in the five most frequent CPHD genes (PROP1, POU1F1, HESX1, LHX3, and LHX4) that was calculated from 21 different CPHD genetic studies.7
A particular strength of our study is the well-characterized cohort of patients with CPHD from a large background population of 2.2M people which corresponds to 40% of the Finnish population. We also evaluated the genetic cohort for a comprehensive set of CPHD/GHD candidate genes identified in the previous years. An inherent limitation to any retrospective study is missing data. The reported frequencies of individual hormone deficiencies were based on the hormone supplementation treatments at the time of data collection and excluded the possibly later developing hormone deficiencies. Our sample size was limited due to the rarity of the disease, which may increase uncertainty in the results. The genetic investigations were conducted using whole exome sequencing, leaving the possibility of undetected regulatory intronic variants or epigenetic factors underlying the disease.
To conclude, congenital CPHD is a rare disease with an estimated incidence of 1 in 16 000 live-born children. Many of its early presenting symptoms, including prolonged neonatal jaundice, severe neonatal hypoglycemia, and reduced height growth, are non-specific, and the diagnosis of the disease is frequently delayed. The combination of reduced growth velocity with a history of neonatal signs of hypopituitarism should be recognized by all clinicians since an early diagnosis of CPHD could even be lifesaving. Also, height growth deviation during the first six months of life should not go unnoticed in any patient since this is a manifestation of severe CPHD phenotype.
Contributors
T.R., P.J.M, and M.H. designed and supervised the study. The literature search was carried out by J.H. and A-P.I. A.T. and H.H. provided resources for the study. Clinical data were collected by J.H., A.T., P.J.M., and H.H., and verified by J.H. and P.J.M. J.H., J.K., A-P.I., K.V., and H.A. analyzed, and J.K., K.V., and T.R. verified the genetic data. The clinical data were interpreted by J.H., P.J.M., M.H., and T.R., and the genetic data by J.H., J.K., A-P.I., K.V., and T.R. J.H., J.K., A-P.I., and K.V. produced the figures and tables. J.H. and K.V. wrote the original draft, and all authors critically revised the manuscript. All authors had access to study data.
Data sharing statement
Study data are not publicly available owing to data privacy issues. Any query can be directed to the corresponding author.
Declaration of interests
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Päivikki and Sakari Sohlberg Foundation (A-P.I.), Biomedicum Helsinki Foundation (JH), and Emil Aaltonen (JH, A-P.I) foundation personal research grants.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101556.
Appendix. Supplementary materials
References
- 1.Higham CE, Johannsson G, Shalet SM. Hypopituitarism. Lancet. 2016;388:2403–2415. doi: 10.1016/S0140-6736(16)30053-8. [DOI] [PubMed] [Google Scholar]
- 2.Prodam F, Caputo M, Mele C, Marzullo P, Aimaretti G. Insights into non-classic and emerging causes of hypopituitarism. Nat Rev Endocrinol. 2021;17:114–129. doi: 10.1038/s41574-020-00437-2. [DOI] [PubMed] [Google Scholar]
- 3.Fang Q, George AS, Brinkmeier ML, et al. Genetics of combined pituitary hormone deficiency: roadmap into the genome era. Endocr Rev. 2016;37:636–675. doi: 10.1210/er.2016-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gundgurthi A, Garg MK, Bhardwaj R, Brar KS, Kharb S, Pandit A. Clinical spectrum of hypopituitarism in India: a single center experience. Indian J Endocrinol Metab. 2012;16:803–808. doi: 10.4103/2230-8210.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regal M, Paramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf) 2001;55:735–740. doi: 10.1046/j.1365-2265.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- 6.Hassan SS, Mukhwana R, Musa S, Ibrahim AAB, Babiker O, Abdullah MA. Aetiologies and clinical patterns of hypopituitarism in Sudanese children. Sudan J Paediatr. 2021;21:53–60. doi: 10.24911/SJP.106-1588448825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Rienzo F, Mellone S, Bellone S, et al. Frequency of genetic defects in combined pituitary hormone deficiency: a systematic review and analysis of a multicentre Italian cohort. Clin Endocrinol (Oxf) 2015;83:849–860. doi: 10.1111/cen.12849. [DOI] [PubMed] [Google Scholar]
- 8.Bas F, Uyguner ZO. Darendeliler F Molecular analysis of PROP1, POU1F1, LHX3, and HESX1 in Turkish patients with combined pituitary hormone deficiency: a multicenter study. Endocrine. 2015;49:479–491. doi: 10.1007/s12020-014-0498-1. [DOI] [PubMed] [Google Scholar]
- 9.Madeira JL, Nishi MY, Nakaguma M, et al. Molecular analysis of brazilian patients with combined pituitary hormone deficiency and orthotopic posterior pituitary lobe reveals eight different PROP1 alterations with three novel mutations. Clin Endocrinol (Oxf) 2017;87:725–732. doi: 10.1111/cen.13430. [DOI] [PubMed] [Google Scholar]
- 10.Gregory LC, Dattani MT. The molecular basis of congenital hypopituitarism and related disorders. J Clin Endocrinol Metab. 2020;105:e2103–e2120. doi: 10.1210/clinem/dgz184. [DOI] [PubMed] [Google Scholar]
- 11.Xatzipsalti M, Voutetakis A, Stamoyannou L, Chrousos GP, Kanaka-Gantenbein C. Congenital hypopituitarism: various genes, various phenotypes. Horm Metab Res. 2019;51:81–90. doi: 10.1055/a-0822-3637. [DOI] [PubMed] [Google Scholar]
- 12.Argente J, Tatton-Brown K, Lehwalder D, Pfäffle R. Genetics of growth disorders - which patients require genetic testing? Front Endocrinol. 2019;10:602. doi: 10.3389/fendo.2019.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulut FD, Özdemir Dilek S, Kotan D, Mengen E, Gürbüz F, Yüksel B. Mutations within the transcription factor PROP1 in a cohort of Turkish patients with combined pituitary hormone deficiency. J Clin Res Pediatr Endocrinol. 2020;12:261–268. doi: 10.4274/jcrpe.galenos.2020.2019.0191. ["VSports" DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen E, Maghnie M, Collot N, et al. Contribution of LHX4 mutations to pituitary deficits in a cohort of 417 unrelated patients. J Clin Endocrinol Metab. 2017;102:290–301. doi: 10.1210/jc.2016-3158. [DOI] [PubMed] [Google Scholar]
- 15.Bertko E, Klammt J, Dusatkova P, et al. Combined pituitary hormone deficiency due to gross deletions in the POU1F1 (PIT-1) and PROP1 genes. J Hum Genet. 2017;62:755–762. doi: 10.1038/jhg.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi JH, Jung CW, Kang E, et al. Rare frequency of mutations in pituitary transcription factor genes in combined pituitary hormone or isolated growth hormone deficiencies in Korea. Yonsei Med J. 2017;58:527–532. doi: 10.3349/ymj.2017.58.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jullien N, Saveanu A, Vergier J, et al. Clinical lessons learned in constitutional hypopituitarism from two decades of experience in a large international cohort. Clin Endocrinol (Oxf) 2021;94:277–289. doi: 10.1111/cen.14355. [DOI] [PubMed] [Google Scholar]
- 18.Gregory LC, Gergics P, Nakaguma M, et al. The phenotypic spectrum associated with OTX2 mutations in humans. Eur J Endocrinol. 2021;185:121–135. doi: 10.1530/EJE-20-1453. ["V体育ios版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynaud R, Albarel F, Saveanu A, et al. Pituitary stalk interruption syndrome in 83 patients: novel HESX1 mutation and severe hormonal prognosis in malformative forms. Eur J Endocrinol. 2011;164:457–465. doi: 10.1530/EJE-10-0892. [V体育ios版 - DOI] [PubMed] [Google Scholar]
- 20.Bashamboo A, Bignon-Topalovic J, Moussi N, McElreavey K, Brauner R. Mutations in the human ROBO1 gene in pituitary stalk interruption syndrome. J Clin Endocrinol Metab. 2017;102:2401–2406. doi: 10.1210/jc.2016-1095. [DOI] [PubMed] [Google Scholar]
- 21.Budny B, Zemojtel T, Kaluzna M, et al. SEMA3A and IGSF10 are novel contributors to combined pituitary hormone deficiency (CPHD) Front Endocrinol. 2020;11:368. doi: 10.3389/fendo.2020.00368. [VSports app下载 - DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes JN, Aubert M, Heatlie J, et al. Identification of an IGSF1-specific deletion in a five-generation pedigree with X-linked central hypothyroidism without macroorchidism. Clin Endocrinol (Oxf) 2016;85:609–615. doi: 10.1111/cen.13094. [DOI] [PubMed] [Google Scholar]
- 23.Hietamäki J, Gregory LC, Ayoub S, et al. Loss-of-function variants in TBC1D32 underlie syndromic hypopituitarism. J Clin Endocrinol Metab. 2020;105:1748–1758. doi: 10.1210/clinem/dgaa078. [DOI (V体育2025版)] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherdel P, Reynaud R, Pietrement C, et al. Priority target conditions for algorithms for monitoring children's growth: interdisciplinary consensus. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranke MB, Wit JM. Growth hormone - past, present and future. Nat Rev Endocrinol. 2018;14:285–300. doi: 10.1038/nrendo.2018.22. [DOI] [PubMed] [Google Scholar]
- 26.Mehta A, Hindmarsh PC, Stanhope RG, et al. The role of growth hormone in determining birth size and early postnatal growth, using congenital growth hormone deficiency (GHD) as a model. ClinEndocrinol(Oxf) 2005;63:223–231. doi: 10.1111/j.1365-2265.2005.02330.x. [DOI] [PubMed] [Google Scholar]
- 27.Leino T, Koskenniemi E, Saranpää P, Strömberg N, Kilpi T. Very high infant vaccination coverage, international ‘false alarms’ had no effect in Finland. Lääkärilehti. 2007;62:739–743. [Google Scholar]
- 28.Mäki P, Wikström K, Hakulinen T, Laatikainen T. 4th Ed. 2017. Health Checkups at Well-Child Clinics: Methodological Handbook; pp. 13–24.VSports手机版 - http://urn.fi/URN:ISBN:978-952-302-964-4 Vol. 2019. 2017;2019:13–24. Available from: Accessed 29 March 2021. [Google Scholar]
- 29.Sankilampi U, Saari A, Laine T, Miettinen PJ, Dunkel L. Use of electronic health records for automated screening of growth disorders in primary care. JAMA. 2013;310:1071–1072. doi: 10.1001/jama.2013.218793. [DOI (VSports app下载)] [PubMed] [Google Scholar]
- 30.Saari A, Sankilampi U, Hannila ML, Kiviniemi V, Kesseli K, Dunkel L. New Finnish growth references for children and adolescents aged 0 to 20 years: Length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann Med. 2011;43:235–248. doi: 10.3109/07853890.2010.515603. ["V体育安卓版" DOI] [PubMed] [Google Scholar]
- 31.Saari A, Sankilampi U, Hannila ML, Saha MT, Mäkitie O, Dunkel L. Screening of turner syndrome with novel auxological criteria facilitates early diagnosis. J Clin Endocrinol Metab. 2012;97:E2125–E2132. doi: 10.1210/jc.2012-1739. [DOI] [PubMed] [Google Scholar]
- 32.Sorva R, Tolppanen EM, Perheentupa J. Variation of growth in length and weight of children. I. Years 1 and 2. Acta PaediatrScand. 1990;79:490–497. doi: 10.1111/j.1651-2227.1990.tb11502.x. [DOI] [PubMed] [Google Scholar]
- 33.Sorva R, Lankinen S, Tolppanen EM, Perheentupa J. Variation of growth in height and weight of children. II. After infancy. Acta PaediatrScand. 1990;79:498–506. doi: 10.1111/j.1651-2227.1990.tb11503.x. [DOI] [PubMed] [Google Scholar]
- 34.Sorva R, Tolppanen EM, Lankinen S, Perheentupa J. Growth evaluation: parent and child specific height standards. Arch Dis Child. 1989;64:1483–1487. doi: 10.1136/adc.64.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kärkinen J, Miettinen PJ, Raivio T, Hero M. Etiology of severe short stature below -3 SDS in a screened Finnish population. Eur J Endocrinol. 2020;183:481–488. doi: 10.1530/EJE-20-0313. ["V体育ios版" DOI] [PubMed] [Google Scholar]
- 36.Wit JM, van Unen H. Growth of infants with neonatal growth hormone deficiency. Arch Dis Child. 1992;67:920–924. doi: 10.1136/adc.67.7.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alt C, Shevell MI, Poulin C, Rosenblatt B, Saint-Martin C, Srour M. Clinical and radiologic spectrum of septo-optic dysplasia: review of 17 cases. J Child Neurol. 2017;32:797–803. doi: 10.1177/0883073817707300. [V体育官网 - DOI] [PubMed] [Google Scholar]
- 38.Thornton PS, Stanley CA, De Leon DD, et al. Recommendations from the pediatric endocrine society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr. 2015;167:238–245. doi: 10.1016/j.jpeds.2015.03.057. ["V体育ios版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodgate P, Jardine LA. Neonatal jaundice: phototherapy. BMJ Clin Evid. 2015;2015:0319. [PMC free article] [PubMed] [Google Scholar]
- 40.Committee on Fetus and Newborn. Adamkin DH. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. 2011;127:575–579. doi: 10.1542/peds.2010-3851. ["VSports最新版本" DOI] [PubMed] [Google Scholar]
- 41.Official Statistics of Finland (OSF): Births [e-publication]. Vol. 2021. Available from: http://www.stat.fi/til/synt/tau_en.htm. Accessed 28 February 2021.
- 42.Boguszewski McdS, Cardoso-Demartini AdA. MANAGEMENT OF ENDOCRINE DISEASE: growth and growth hormone therapy in short children born preterm. Eur J Endocrinol. 2017;176:R111–R122. doi: 10.1530/EJE-16-0482. [V体育安卓版 - DOI] [PubMed] [Google Scholar]
- 43.Euser AM, de Wit CC, Finken MJJ, Rijken M, Wit JM. Growth of preterm born children. Horm Res. 2008;70:319–328. doi: 10.1159/000161862. [DOI] [PubMed] [Google Scholar]
- 44.Finnish Institute for Health and Welfare. Renewal of Children's Growth Monitoring. Expert Group Report.. Vol. 2021. Available from: http://urn.fi/URN:NBN:fi-fe201205085159. Accessed 29 March 2021.
- 45.Sulonen AM, Ellonen P, Almusa H, et al. Comparison of solution-based exome capture methods for next generation sequencing. Genome Biol. 2011;12 doi: 10.1186/gb-2011-12-9-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherry ST, Ward M, Sirotkin K. dbSNP-database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res. 1999;9:677–679. [PubMed] [Google Scholar]
- 48.Institute for Molecular Medicine Finland (FIMM) University of Helsinki Finland. Sequencing Initiative Suomi project (SISu) v4.1. Available from: http://sisuproject.fi (V体育安卓版). Accessed 17 May 2021.
- 49.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med Off J Am Coll Med Genet. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, InterVar Wang K. Clinical Interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am J Hum Genet. 2017;100:267–280. doi: 10.1016/j.ajhg.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohva E, Huopio H, Hietamäki J, Hero M, Miettinen PJ, Raivio T. Treatment of gonadotropin deficiency during the first year of life: long-term observation and outcome in five boys. Hum Reprod. 2019;34:863–871. doi: 10.1093/humrep/dez040. ["V体育2025版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward DJ, Connolly DJA, Griffiths PD. Review of the MRI brain findings of septo-optic dysplasia. Clin Radiol. 2021;76:160.e1–160.e14. doi: 10.1016/j.crad.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Kelberman D, Turton JP, Woods KS, et al. Molecular analysis of novel PROP1 mutations associated with combined pituitary hormone deficiency (CPHD) Clin Endocrinol (Oxf) 2009;70:96–103. doi: 10.1111/j.1365-2265.2008.03326.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Zhang X, Long R, Yu L. A novel deletion mutation of the SOX2 gene in a child of Chinese origin with congenital bilateral anophthalmia and sensorineural hearing loss. J Genet. 2018;97:1007–1011. [PubMed] [Google Scholar]
- 55.Jiman OA, Taylor RL, Lenassi E, et al. Diagnostic yield of panel-based genetic testing in syndromic inherited retinal disease. Eur J Hum Genet EJHG. 2020;28:576–586. doi: 10.1038/s41431-019-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel L, McNally RJ, Harrison E, Lloyd IC, Clayton PE. Geographical distribution of optic nerve hypoplasia and septo-optic dysplasia in Northwest England. J Pediatr. 2006;148:85–88. doi: 10.1016/j.jpeds.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 57.Lacey KA, Parkin JM. Causes of short stature. A community study of children in Newcastle upon Tyne. Lancet. 1974;1:42–45. doi: 10.1016/s0140-6736(74)93041-4. [DOI] [PubMed] [Google Scholar]
- 58.Rona RJ, Tanner JM. Aetiology of idiopathic growth hormone deficiency in England and Wales. Arch Dis Child. 1977;52:197–208. doi: 10.1136/adc.52.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vimpani GV, Vimpani AF, Lidgard GP, Cameron EH, Farquhar JW. Prevalence of severe growth hormone deficiency. Br Med J. 1977;2:427–430. doi: 10.1136/bmj.2.6084.427. [DOI (VSports手机版)] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. JPediatr. 1994;125:29–35. doi: 10.1016/s0022-3476(94)70117-2. [DOI] [PubMed] [Google Scholar]
- 61.Bosch I Ara L, Katugampola H, Dattani MT. Congenital hypopituitarism during the neonatal period: epidemiology, pathogenesis, therapeutic options, and outcome. Front Pediatr. 2021;8 doi: 10.3389/fped.2020.600962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaleva M, Toppari J. Cryptorchidism: an indicator of testicular dysgenesis? Cell Tissue Res. 2005;322:167–172. doi: 10.1007/s00441-005-1143-3. [DOI] [PubMed] [Google Scholar]
- 63.Hansen TWR, Maisels MJ, Ebbesen F, et al. Sixty years of phototherapy for neonatal jaundice - from serendipitous observation to standardized treatment and rescue for millions. J Perinatol Off J Calif Perinat Assoc. 2020;40:180–193. doi: 10.1038/s41372-019-0439-1. ["VSports app下载" DOI] [PubMed] [Google Scholar]
- 64.Hay WW, Jr, Raju TN, Higgins RD, Kalhan SC, Devaskar SU. Knowledge gaps and research needs for understanding and treating neonatal hypoglycemia: workshop report from Eunice Kennedy Shriver National Institute of Child Health and Human Development. J Pediatr. 2009;155:612–617. doi: 10.1016/j.jpeds.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nebesio TD, McKenna MP, Nabhan ZM, Eugster EA. Newborn screening results in children with central hypothyroidism. J Pediatr. 2010;156:990–993. doi: 10.1016/j.jpeds.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 66.Cavarzere P, Biban P, Gaudino R, et al. Diagnostic pitfalls in the assessment of congenital hypopituitarism. J Endocrinol Invest. 2014;37:1201–1209. doi: 10.1007/s40618-014-0139-9. [DOI] [PubMed] [Google Scholar]
- 67.Mehta S, Brar PC. Severe, persistent neonatal hypoglycemia as a presenting feature in patients with congenital hypopituitarism: a review of our case series. J Pediatr Endocrinol Metab JPEM. 2019;32:767–774. doi: 10.1515/jpem-2019-0075. [DOI] [PubMed] [Google Scholar]
- 68.Webb EA, Dattani MT. Septo-optic dysplasia. Eur J Hum Genet EJHG. 2010;18:393–397. doi: 10.1038/ejhg.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gascoin-Lachambre G, Brauner R, Duche L, Chalumeau M. Pituitary stalk interruption syndrome: diagnostic delay and sensitivity of the auxological criteria of the growth hormone research society. PLoS One. 2011;6:e16367. doi: 10.1371/journal.pone.0016367. ["V体育平台登录" DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorbenko del Blanco D, de Graaff LC, Visser TJ, Hokken-Koelega AC. Single-nucleotide variants in two Hedgehog genes, SHH and HHIP, as genetic cause of combined pituitary hormone deficiency. Clin Endocrinol (Oxf) 2013;78:415–423. doi: 10.1111/cen.12000. [DOI] [PubMed] [Google Scholar]
- 71.Roessler E, Belloni E, Gaudenz K, et al. Mutations in the C-terminal domain of Sonic Hedgehog cause holoprosencephaly. Hum Mol Genet. 1997;6:1847–1853. doi: 10.1093/hmg/6.11.1847. [DOI] [PubMed] [Google Scholar]
- 72.Solomon BD, Mercier S, Vélez JI, et al. Analysis of genotype-phenotype correlations in human holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:133–141. doi: 10.1002/ajmg.c.30240. ["V体育2025版" DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jee YH, Gangat M, Yeliosof O, et al. Evidence that the etiology of congenital hypopituitarism has a major genetic component but is infrequently monogenic. Front Genet. 2021;12 doi: 10.3389/fgene.2021.697549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnhold IJ, Franca MM, Carvalho LR, Mendonca BB, Jorge AA. Role of GLI2 in hypopituitarism phenotype. J Mol Endocrinol. 2015;54:R141–R150. doi: 10.1530/JME-15-0009. [DOI (V体育官网)] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.