V体育安卓版 - Abstract
In many branches of medicine, treatment is guided by measuring its effects on underlying physiology. In this regard, the efficacy of rehabilitation/recovery therapies could be enhanced if their administration was guided by measurements that directly capture treatment effects on neural function. Measures of brain function via EEG may be useful towards this goal and have advantages such as ease of bedside acquisition, safety, and low cost. This review synthetizes EEG studies during the subacute phase post-stroke, when spontaneous recovery is maximal, and focuses on movement. Event-related measures reflect cortical activation and inhibition, while connectivity measures capture function of cortical networks. Several EEG-based measures are related to motor outcomes post-stroke and warrant further evaluation VSports最新版本. Ultimately, they may be useful for clinical decision-making and clinical trial design in stroke neurorehabilitation.
"V体育官网" Introduction
Stroke is a major cause of long-term disability. Motor deficits are persistent in 50–75% of cases and are a major contributor to post-stroke impairment and disability. 1 Acute reperfusion therapies can improve functional outcomes with ischemic stroke, but only 20% of patients in the US get treated,2 and many nevertheless have long-term disability. As a result, increasing attention is being devoted to therapies that promote neural repair, particularly those initiated during the early days-weeks post-stroke given their potential for greater effect in this period. 3 These points underscore the need for an improved understanding of brain plasticity during subacute stroke, as well as the pressing need for validated biomarkers that capture brain activity in a manner that can stratify patients and inform clinical decision-making V体育平台登录. The current review considers this with a focus on EEG applications during motor recovery post-stroke. EEG is attractive because it can be acquired at the bedside in complex medical settings, safely, with low cost and excellent temporal resolution. Together, these qualities suggest that EEG is a promising tool for understanding and treating patients with stroke.
The metrics provided by EEG reflect the temporal and spatial summation of excitatory and inhibitory post-synaptic dendritic potentials of pyramidal neurons belonging mainly to layers III and V of the cortex. EEG signals therefore have amplitude and frequency characteristics that vary over time. Recording from the scalp places limits on the spatial resolution with which signals can be localized neuroanatomically, which can be partially offset using source analysis. EEG can be recorded at rest, though this does not capture events underlying movement generation, or it can be recorded during motor task performance, which provides insight into movement planning and execution and so is the current focus. Task-related EEG measures the activity within and between cortical areas related to motor control VSports注册入口. Longitudinal recordings capture the neural plasticity underlying stroke recovery and have the potential to inform the timing, dose, and duration of rehabilitation/recovery therapies in individual patients.
Event-Related Desynchronization and Synchronization
Event-related desynchronization and synchronization are measures derived from EEG recordings that are used to study cortical activity and inhibition, respectively.
At rest, neurons within a cortical area show substantial synchronous activity. During unilateral voluntary movements, neurons involved in the task become desynchronized, decreasing EEG power, predominantly in sensorimotor cortex contralateral to movement. This reduction in task-related spectral power (TR-pow) reflects cortical activation,4,5 a phenomenon known as event-related desynchronization (ERD). ERD begins 1–2 seconds before the onset of, and persists during, a spontaneous voluntary movement6, and is mainly in the beta frequency band (≈13–30Hz), suggesting that the cortical activity changes in this frequency band reflect cortical activation and corticospinal excitability changes involved in the initiation and execution of voluntary movements. 5,7 As cortical activity is calculated in slightly different ways in the literature (TR-pow or ERD), we will refer to these two metrics as cortical activity VSports在线直播.
The return of cortical activity to a resting state can also be measured. Also predominantly in the beta frequency band, EEG power increases (compared with baseline) at the end of a movement, predominantly in the sensorimotor areas contralateral to movement. 6 This phenomenon, known as event-related synchronization (ERS), corresponds to a return to the basal state, reflecting deactivation and active inhibition. 6,8 ERD and ERS manifest in different frequency bands in different contexts, providing additional insights: in the theta frequency band (4–8Hz), with high cognitive involvement; in the alpha frequency band (8–12Hz), with high attention involvement;9 and in the gamma frequency band (30–90Hz), with intense motor contraction. 10 Use of brain cognitive resources varies with choice of motor task,11 and so calculation of ERD and ERS in multiple frequency bands provides a measure of cognitive network engagement in controlling motor strategy. These measures enable a robust understanding of brain function after stroke and its change over time related to neuroplasticity V体育2025版.
Motor control of the contralesional (paretic) upper extremity:
Many forms of brain mapping have found underactivation of the ipsilesional hemisphere in subacute stroke patients. Similarly, using EEG, cortical activity in ipsilesional sensorimotor cortex is reduced compared to healthy subjects, during premovement12,13 and movement of the paretic hand/finger12–14, and compared to the contralesional hemisphere during movement15,16 (Figure 1). This stroke-related decrease in cortical activity is well-established in the alpha and beta frequency bands and more recently in the theta band during premovement. 12 During grip tasks, decreased ipsilesional sensorimotor and parietal cortex activity is present acutely, during the initial days post-stroke12, and persists into the subacute phase at 1, 2, 3 and 4 months after stroke. 12,16 This reduction in cortical activity may be a direct consequence of cortical injury or it may be an indirect effect from network-wide diaschisis. 12 EEG has advantages for capturing diaschisis:17 cortical oscillations depend on local and remote connections between neurons,18 and so a decrease in sensorimotor cortex activity may be due to injury elsewhere in the motor network. 16 A role for diaschisis is suggested by the observed negative correlation between total infarct volume and sensorimotor cortical activity. 19 Increased tonic inhibition at the peri-infarct zone could also contribute to a decrease in regional cortical activity VSports. 20.
Figure 1:
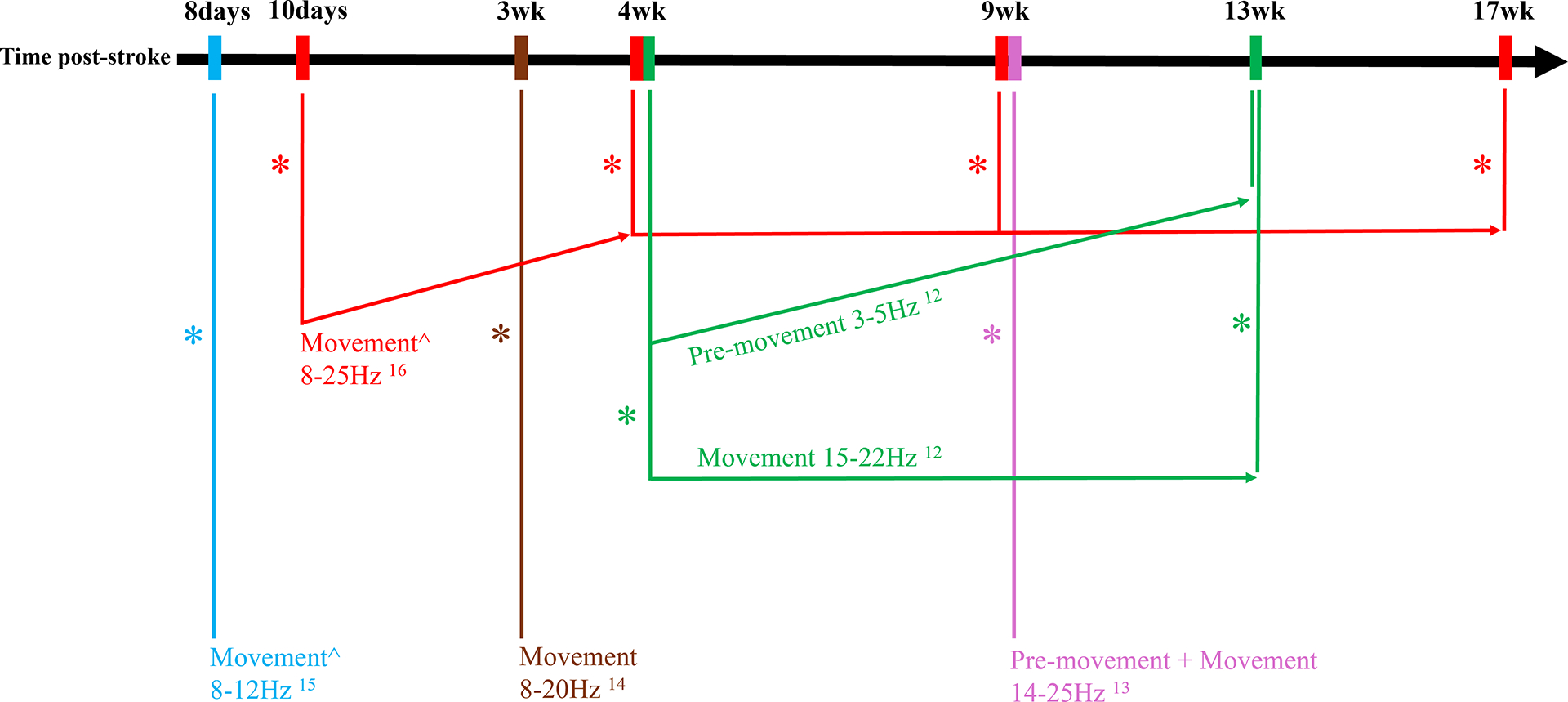
Summary of studies examining ipsilesional cortical activity in subacute stroke during a motor task VSports app下载. All these studies observed decreased ipsilesional cortical activity compared to healthy controls, except where there is ^, representing a comparison with the contralesional hemisphere. The * represents significantly lower ipsilesional cortical during pre-movement and/or movement, as specified. Results are represented according to the time post-stroke (x-axis). Non-horizontal arrows represent significant within-subject longitudinal changes. Numbers represent the references associated with the results.
Motor control of the ipsilesional (non-paretic) upper extremity:
Some authors have studied cortical activity when movement is performed by an ipsilesional (non-paretic) finger. In this case, ipsilesional sensorimotor cortex shows more activity than when the movement is performed by a contralesional (paretic) finger.15 This suggests relative preservation of the ipsilesional hemisphere control of ipsilateral movements, despite the stroke, though this finding may reflect use of separate neural resources for moving each hand.21
EEG changes during stroke recovery:
Longitudinal EEG-based assessments capture changes in brain function associated with spontaneous motor recovery after stroke. The decrease in ipsilesional sensorimotor cortex activity seen in the alpha and beta frequency bands returns towards normal between 10 days and 1 month post-stroke, compared with activity in the contralesional hemisphere (Fig. 1).16 Conversely, contralesional sensorimotor activity tends to decrease with recovery, reflecting disengagement of the contralesional hemisphere.16 On average, ipsilesional sensorimotor and parietal activity plateaus after 1 month, remaining lower than values in healthy controls.12,16 Lower frequency oscillations within ipsilesional sensorimotor and parietal cortex decrease acutely and then increase 1–3 months post-stroke, reaching values comparable to those of healthy controls.12 These EEG changes over time may reflect a range of processes related to neural plasticity, such as axonal sprouting or greater activity of secondary motor cortices within motor networks.16
Relation to motor deficits:
Higher ipsilesional motor cortex activity, captured as larger alpha- and beta-ERD during paretic wrist movement 1.5 months post-stroke, correlates with smaller motor deficits (Fugl-Meyer score, grip force, and Nine-Hole Peg test).19 Furthermore, increases over time in ipsilesional sensorimotor and parietal cortical activity during paretic hand movement are associated with extent of motor recovery (Fugl-Meyer score and Box and Block test).12,16 Such findings are promising, and further studies are needed to evaluate the performance of these EEG measures as biomarkers of post-stroke cortical plasticity.3,12,16
On the other hand, higher ipsilesional post-movement inhibition, captured as larger ERS during hand grip 4 months post-stroke, correlates with larger motor deficits.8 Poorer motor status may be a consequence of greater ipsilesional cortical inhibition. Theoretically, training on a brain-computer interface to reduce ERS may improve motor outcomes.8
These studies rely on correlation and so do not establish causation, i.e., whether these electrophysiological findings are the cause or consequence of motor deficits.12,16,22 This could be evaluated by creating a virtual lesion using TMS, as has been done with fMRI findings. Measuring cortical activity and motor function frequently during recovery could also address causality by examining the temporality of EEG changes relative to behavioral improvement.22
"V体育安卓版" Functional connectivity
Measuring activity in different cortical areas during movement can be used to study their functional interactions, beyond their anatomical links.23 Towards this, functional connectivity between two cortical areas can be measured using EEG coherence, which estimates the consistency of amplitude and phase between any signal pair in a particular frequency band.24
The first EEG connectivity studies observed that, during voluntary muscle contraction, functional connectivity increased between premotor cortex (PMC), primary motor cortex (M1) and supplementary motor areas (SMA) contralateral to movement, in the alpha and beta frequency bands.23,25 That this was seen during movement preparation and execution23,25 echoes anatomic relationships between these cortical areas26. Other studies specifically examined causal relationships within a predefined functional network using effective connectivity to model influences of one region on another;27 this approach is promising but has received less attention. The high temporal resolution of EEG allows examination of cortical network interactions preceding and during movement using event-related coherence,28 which provides information that may be useful to target when as well as where brain function may be therapeutically modulated, such as by non-invasive brain stimulation.
"V体育ios版" Global network reorganization:
One way to study global functional connectivity is via graph theory. This method evaluates the efficiency of overall brain function by quantifying the interconnectivity and redundancy of connections. At 1 month post-stroke, patients had functional brain network segregation, with key regions not interconnected, as well as redundant connections, suggesting that stroke reduces local efficiency of cortical areas.29
"V体育平台登录" Ipsilesional hemisphere connectivity and motor function:
Studies have examined functional connectivity of ipsilesional areas involved in motor control (Fig. 2), e.g., M1, SMA, PMC, and anterior intraparietal sulcus (aIPS).
Figure 2:
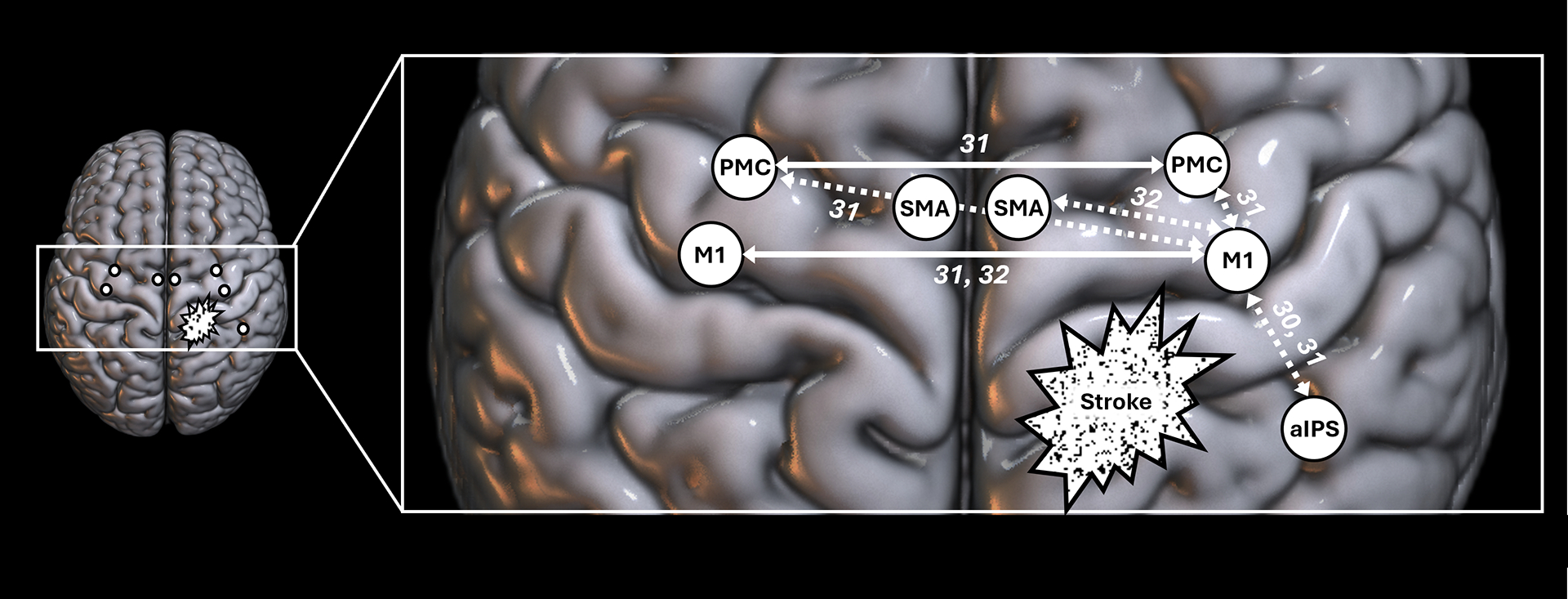
Summary of the link between motor deficits and cortical coherence after stroke. Solid arrows represent a positive link between cortical coherence and motor performance, indicating that higher coherence levels are associated with better motor status, while dotted arrows represent a negative link between cortical coherence and motor performance, indicating that higher coherence levels are associated with poorer motor performance. The numbers are references associated with each result. aIPS=anterior Intraparietal Sulcus, M1=Primary Motor Cortex, PMC=Premotor Cortex, SMA=Supplementary Motor Area.
Patients with subacute stroke show higher M1-aIPS connectivity during paretic movements compared to healthy controls,30 suggesting increased integrative demand post-stroke, here specifically reflecting the role of aIPS in visuomotor and sensorimotor integration. Furthermore, in subacute stroke patients, stronger ipsilesional connectivity (alpha- and beta-connectivity between M1-PMC31 and between M1-aIPS30) is associated with greater motor deficits (Fugl-Meyer, Nine-Hole Peg, and grip strength). Ipsilesional connectivity also predicts future motor status, as higher connectivity between M1-PMC at 1-month31 and M1-SMA at 2-months post-stroke32 correlates negatively with extent of subsequent motor recovery at 2 and 3 months, respectively. This may be a form of maladaptive plasticity; Li et al32 speculate that these increases in ipsilesional connectivity interfere with contralesional contributions, thereby impeding recovery. Subacute ipsilesional connectivity warrants further study as a potential biomarker of the cortical processes underlying motor status and its recovery over time.
Inter-hemispheric connectivity and motor function:
Conversely, stronger inter-hemispheric connectivity between homologous areas is associated with smaller motor deficits (Fig. 2), e.g., alpha- and beta-connectivity between M1-M1 and PMC-PMC correlates positively with Fugl-Meyer score.31 Further, change in M1-M1 alpha-connectivity between 2 and 3 months post-stroke correlates with extent of motor recovery.32 One interpretation is that inter-hemispheric connectivity between homologous motor areas supports motor recovery, an idea that requires further study. However, inter-hemispheric connectivity between non-homologous areas is associated with larger motor deficits, e.g., ipsilesional M1-contralesional PMC connectivity in the beta band correlates negatively with Fugl-Meyer score.31 This type of connectivity might therefore reflect dysfunctional attempts at communication between hemispheres.
Limits and perspectives
Strengths include that ipsilesional ERD studies are numerous and convergent. Contralesional hemisphere activity changes15 and ipsilesional control of the non-paretic hand33 provide insights into motor system function after stroke and warrant additional investigation. Few studies have examined serial EEG in a clinical trial setting, e.g., during telerehabilitation,34 though this approach is promising. Candidate EEG-based biomarkers require further study, e.g., of their psychometric properties and their performance across different patient subgroups. A key limitation is that EEG captures cortical signals and so is less sensitive to subcortical processes.
EEG biomarkers have the potential to guide neurorehabilitation clinical practice/research, much as CT/MRI is used in acute stroke. EEG acquired during performance of a motor task provides detailed insights into function and plasticity of cortical networks; different tasks can be used, depending on a patient’s motor status, including a simple motor task such as gripping or tapping, a complex multi-joint movement with large amplitude such as targeted reaching, or a task with high functional ecology such as bringing a cup to the mouth or driving. Medical therapies are often guided by measuring their effects on the target physiological system, e.g., serum TSH levels are used to guide levothyroxine treatment of hypothyroidism. In this context, several EEG measures hold promise as tools to measure brain function during the early weeks post-stroke when the brain is maximally galvanized for plasticity. The low cost, safety, and ease of use of EEG at the bedside enable frequent recordings, which stand to monitor cortical function and its plasticity in the stroke rehabilitation setting in much the same way that an EKG is used to monitor cardiac function in the ICU.
Funding Sources
NIH R01NR015591
Abbreviations
- aIPS
anterior intraparietal sulcus
- ERD
event-related desynchronization
- ERS
event-related synchronization
- M1
primary motor cortex
- PMC
premotor cortex
- SMA
supplementary motor area
"VSports在线直播" Footnotes
Disclosures
Dr. Cramer serves as a consultant for Constant Therapeutics, BrainQ, Myomo, MicroTransponder, Elevian, Panaxium, Beren Therapeutics, Medtronic, Stream Biomedical, NeuroTrauma Sciences, and TRCare.
References
- 1.Anwer S, Waris A, Gilani SO, Iqbal J, Shaikh N, Pujari AN, Niazi IK. Rehabilitation of Upper Limb Motor Impairment in Stroke: A Narrative Review on the Prevalence, Risk Factors, and Economic Statistics of Stroke and State of the Art Therapies. Healthcare. 2022;10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinaldo L, Cloft HJ, Rangel Castilla L, Rabinstein AA, Brinjikji W. Utilization rates of tissue plasminogen activator and mechanical thrombectomy in patients with acute stroke and underlying malignancy. J. Neurointerventional Surg 2019;11:768–771. ["V体育官网" DOI] [PubMed] [Google Scholar]
- 3.Cramer SC. Recovery After Stroke. Contin. Minneap. Minn 2020;26:415–434. [DOI] [PubMed] [Google Scholar]
- 4.Pfurtscheller G. Functional brain imaging based on ERD/ERS. Vision Res. 2001;41:1257–1260. [V体育安卓版 - DOI] [PubMed] [Google Scholar]
- 5.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol 1999;110:1842–1857. [V体育官网入口 - DOI] [PubMed] [Google Scholar]
- 6.Monge-Pereira E, Molina-Rueda F, Rivas-Montero FM, Ibáñez J, Serrano JI, Alguacil-Diego IM, Miangolarra-Page JC. Electroencephalography as a post-stroke assessment method: An updated review. Neurol. Engl. Ed 2017;32:40–49. [DOI] [PubMed] [Google Scholar]
- 7.Takemi M, Masakado Y, Liu M, Ushiba J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J. Neurophysiol 2013;110:1158–1166. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser V, Daly I, Pichiorri F, Mattia D, Müller-Putz GR, Neuper C. Relationship Between Electrical Brain Responses to Motor Imagery and Motor Impairment in Stroke. Stroke. 2012;43:2735–2740. [DOI] [PubMed] [Google Scholar]
- 9.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev 1999;29:169–195. ["VSports" DOI] [PubMed] [Google Scholar]
- 10.Brown P, Salenius S, Rothwell JC, Hari R. Cortical Correlate of the Piper Rhythm in Humans. J. Neurophysiol 1998;80:2911–2917. [DOI] [PubMed] [Google Scholar]
- 11.Lin DJ, Erler KS, Snider SB, Bonkhoff AK, DiCarlo JA, Lam N, Ranford J, Parlman K, Cohen A, Freeburn J, et al. Cognitive Demands Influence Upper Extremity Motor Performance During Recovery From Acute Stroke. Neurology. 2021;96:e2576–e2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bönstrup M, Krawinkel L, Schulz R, Cheng B, Feldheim J, Thomalla G, Cohen LG, Gerloff C. Low‐Frequency Brain Oscillations Track Motor Recovery in Human Stroke. Ann. Neurol 2019;ana.25615. [DOI (VSports注册入口)] [PubMed] [Google Scholar]
- 13.Platz T. Multimodal EEG analysis in man suggests impairment-specific changes in movement-related electric brain activity after stroke. Brain. 2000;123:2475–2490. [V体育官网 - DOI] [PubMed] [Google Scholar]
- 14.Pfurtscheller G, Aranibar A, Wege W. Changes in central EEG activity in relation to voluntary movement. II. Hemiplegic patients. Prog. Brain Res 1980;54:491–495. ["V体育安卓版" DOI] [PubMed] [Google Scholar]
- 15.Stępień M, Conradi J, Waterstraat G, Hohlefeld FU, Curio G, Nikulin VV. Event-related desynchronization of sensorimotor EEG rhythms in hemiparetic patients with acute stroke. Neurosci. Lett 2011;488:17–21. [DOI] [PubMed] [Google Scholar]
- 16.Tangwiriyasakul C, Verhagen R, Rutten WLC, van Putten MJAM. Temporal evolution of event-related desynchronization in acute stroke: A pilot study. Clin. Neurophysiol 2014;125:1112–1120. [DOI] [PubMed] [Google Scholar]
- 17.Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137:2408–2422. [V体育平台登录 - DOI] [PubMed] [Google Scholar]
- 18.Jones SR, Pinto DJ, Kaper TJ, Kopell N. Alpha-frequency rhythms desynchronize over long cortical distances: a modeling study. J. Comput. Neurosci 2000;9:271–291. ["V体育平台登录" DOI] [PubMed] [Google Scholar]
- 19.Bartur G, Pratt H, Soroker N. Changes in mu and beta amplitude of the EEG during upper limb movement correlate with motor impairment and structural damage in subacute stroke. Clin. Neurophysiol 2019;130:1644–1651. [DOI] [PubMed] [Google Scholar]
- 20.Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST. Reducing excessive GABAergic tonic inhibition promotes post-stroke functional recovery. Nature. 2010;468:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of Distinct Motor Cortex Regions During Ipsilateral and Contralateral Finger Movements. J. Neurophysiol 1999;81:383–387. [DOI] [PubMed] [Google Scholar]
- 22.Cassidy JM, Mark JI, Cramer SC. Functional connectivity drives stroke recovery: shifting the paradigm from correlation to causation. Brain J. Neurol 2022;145:1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rappelsberger P, Pfurtscheller G, Filz O. Calculation of event-related coherence?A new method to study short-lasting coupling between brain areas. Brain Topogr. 1994;7:121–127. ["V体育官网入口" DOI] [PubMed] [Google Scholar]
- 24.Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J. Neurosci. Methods 2007;166:41–52. ["VSports app下载" DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerloff C. Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain. 1998;121:1513–1531. [DOI (VSports手机版)] [PubMed] [Google Scholar]
- 26.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. Off. J. Soc. Neurosci 1991;11:667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. [DOI (VSports)] [PubMed] [Google Scholar]
- 28.Lui KK, Nunez MD, Cassidy JM, Vandekerckhove J, Cramer SC, Srinivasan R. Timing of readiness potentials reflect a decision-making process in the human brain. Comput. Brain Behav 2021;4:264–283. [DOI (VSports)] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J, Xi X, Wang T, Wang J, Li L, Lü Z. Analysis of corticomuscular-cortical functional network based on time-delayed maximal information spectral coefficient. J. Neural Eng 2023;20:056017. [DOI] [PubMed] [Google Scholar]
- 30.Bönstrup M, Schulz R, Schön G, Cheng B, Feldheim J, Thomalla G, Gerloff C. Parietofrontal network upregulation after motor stroke. NeuroImage Clin. 2018;18:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoshino T, Oguchi K, Inoue K, Hoshino A, Hoshiyama M. Relationship between upper limb function and functional neural connectivity among motor related-areas during recovery stage after stroke. Top. Stroke Rehabil 2020;27:57–66. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Li S, Roh J, Wang C, Zhang Y. Multimodal Neuroimaging Using Concurrent EEG/fNIRS for Poststroke Recovery Assessment: An Exploratory Study. Neurorehabil. Neural Repair 2020;34:1099–1110. [DOI] [PubMed] [Google Scholar]
- 33.Graziadio S, Tomasevic L, Assenza G, Tecchio F, Eyre JA. The myth of the ‘unaffected’ side after unilateral stroke: Is reorganisation of the non‐infarcted corticospinal system to re-establish balance the price for recovery? Exp. Neurol 2012;238:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J, Quinlan EB, Dodakian L, McKenzie A, Kathuria N, Zhou RJ, Augsburger R, See J, Le VH, Srinivasan R, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015;138:2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]


