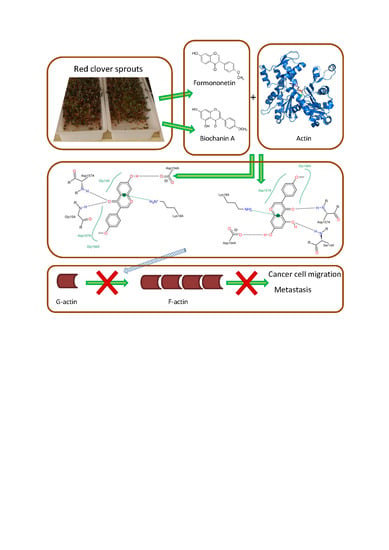
Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells
"> Figure 1
Depiction (in 2D) of the main interactions established between the active site of actin and (a) genistein; (b) formononetin; (c) daidzein; (d) biochanin A. Continuous lines represent hydrophobic interactions, while dashed lines show hydrogen bonds and dashed lines connected to the bond between ring A and C represent π-π interactions.
"> Figure 2ITC raw data from the titration of actin with red clover sprout extracts cultivated under (a) white light for 10 days; (b) UVA 3 for days; (c) UVA for 8 days; (d) UVB for 11 days.
">
Abstract
: Actin functions are crucial for the ability of the cell to execute dynamic cytoskeleton reorganization and movement. Nutraceuticals that form complexes with actin and reduce its polymerization can be used in cancer therapy to prevent cell migration and metastasis of tumors. The aim of this study was to evaluate the ability of isoflavones to form complexes with actin. Docking simulation and isothermal titration calorimetry were used for this purpose. The formation of complexes by hydrogen bonds, hydrophobic and π-π interactions was demonstrated. Interactions occurred at the ATP binding site, which may limit the rotation of the actin molecule observed during polymerization and also at the site responsible for contacts during polymerization, reducing the ability of the molecule to form filaments. The greatest therapeutic potential was demonstrated by isoflavones occurring in red clover sprouts, i V体育官网入口. e. , biochanin A and formononetin, being methoxy derivatives of genistein and daidzein. Keywords: actin; cell migration; isoflavones; anti-metastatic activity .1. Introduction
2. Results and Discussion
2.1. Concentration of Isoflavones in Red Clover Sprouts
2.2. Characterisation of Complexes of Isoflavones with Actin
2.3. Energetic Effects of Actin-Isoflavones Interactions
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Isothermal Titration Calorimetry
3.3. Cultivation of Sprouts
3.4. Extraction and LC-ESI-MS Analysis of Isoflavones
3.5. Molecular Modelling
3.6. Statistical Analysis
"V体育安卓版" 4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
VSports最新版本 - References
- Belmont, L.D.; Orlova, A.; Drubin, D.G.; Egelman, E.H. A change of actin conformation associated with filament instability after Pi release. Proc. Natl. Acad. Sci. USA 1999, 96, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Rouabhia, D.; Park, H.J.; Giasson, L.; Zhang, Z. Effect of soft foods on primary human gingival epithelial cell growth and the wound healing process. Food Res. Int. 2017, 100, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-H.; Ho, C.-T.; Chen, Z.-F.; Chen, L.-C.; Whang-Peng, J.; Lin, T.-N.; Ho, Y.-S. The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J. Food Drug Anal. 2018, 26, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, A.; Jung, W.K.; Jeon, T.J. Effect of fucoidan on cell morphology and migration in osteoblast. Food Sci. Biotechnol. 2015, 24, 699–704. [Google Scholar] [CrossRef]
- Reutzel, R.; Yoshioka, C.; Govindasamy, L.; Yarmola, E.G.; Agbandje-McKenna, M.; Bubb, M.R.; McKenna, R. Actin crystal dynamics: Structural implications for F-actin nucleation, polymerization, and branching mediated by the anti-parallel dimer. J. Struct. Biol. 2004, 146, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Dedova, I.V.; Dedov, V.N.; Nosworthy, N.J.; Hambly, B.D.; Remedios, C.G. Cofilin and Dnase I affect the conformation of the small domain of actin. Biophys. J. 2002, 82, 3134–3143. [Google Scholar] [CrossRef]
- Kienchin, V.A.; King, R.; Tanaka, J.; Marriott, G.; Rayment, I. Structural basis of swinholide A binding to actin. Chem. Biol. 2005, 12, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Brayford, S.; Schevzov, G.; Vos, J.; Gunning, P. The role of actin cytoskeleton in cancer and its potential use as a therapeutic target. In The Cytoskeleton in Health and Disease; Schatten, H., Ed.; Springer Science+Business Media: New York, NY, USA, 2015; pp. 373–391. ISBN 978-1-4939-2903-0. [Google Scholar]
- Pawlik, A.; Szczepański, M.A.; Klimaszewska, A.; Gackowska, L.; Zuryn, A.; Grzanka, A. Phenethyl isothiocyanate-induced cytoskeleton changes and cell death in lung cancer cell. Food Chem. Toxicol. 2012, 50, 3577–3594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Z.; Cao, F.K.; Du, S.W. The apoptotic pathways in the curcumin analog MHMD-induced lung cancer cell death and the essential role of actin polymerization during apoptosis. Biomed. Pharmacother. 2015, 71, 128–134. ["V体育2025版" Google Scholar] [CrossRef] [PubMed]
- Böhl, M.; Tietze, S.; Sokoll, A.; Madathil, S.; Pfennig, F.; Apostolakis, J.; Fahmy, K.; Gutzeit, H.O. Flavonoids affect actin functions in cytoplasm and nucleus. Biophys. J. 2007, 93, 2767–2780. [Google Scholar] [CrossRef] [PubMed]
- Miralles, F.; Visa, N. Actin in transcription and transcription regulation. Curr. Opin. Cell Biol. 2006, 18, 261–299. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Z.; Wang, L.; Deng, L.; Sun, J.; Jiang, X.; Zhang, Y.; Tian, L.; Wag, Y.; Bai, W. Scandenolone, a natural isoflavone derivative from Cudrania tricuspidata fruit, targets EGFR to induce apoptosis and block autophagy flux in human melanoma cells. J. Funct. Foods 2017, 37, 229–240. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Wang, D.; Fang, F.; Lai, J.; Wu, T.; Tsao, R. Isoflavone, γ-aminobutyric acid contents and antioxidant activities are significantly increased during germination of three Chinese soybean cultivars. J. Funct. Foods 2015, 14, 596–604. [Google Scholar] [CrossRef]
- Budryn, G.; Gałązka-Czarnecka, I.; Brzozowska, E.; Grzelczyk, J.; Mostowski, R.; Żyżelewicz, Ż.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H. Evaluation of estrogenic activity of red clover (Trifolium pratense L.) sprouts cultivated under different conditions by content of isoflavones, calorimetric study and molecular modeling. Food Chem. 2018, 245, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Pałecz, B.; Rachwal-Rosiak, D.; Oracz, J.; Zaczyńska, D.; Sylwia, B.; Inmaculada, N.-G.; Josefina María, V.M.; Horacio, P.-S. Effect of inclusion of hydroxycinnamic and chlorogenic acids from green coffee bean in beta-cyclodextrin on their interactions with whey, egg white and soy protein isolates. Food Chem. 2015, 168, 276–287. ["V体育安卓版" Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.M.; Wähälä, K.; Adlercreutz, H. Identification of urinary metabolites of the red clover isoflavones formononetin and biochanin A in human subjects. J. Agric. Food Chem. 2004, 52, 6802–6809. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G. A review of dietary polyphenol-plasma protein interactions: Characterization, influence on the bioactivity, and structure-affinity relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Böhl, M.; Czupalla, C.; Tokalov, S.V.; Hoflack, B.; Gutzeit, H.O. Identification of actin as quercetin-binding protein: An approach to identify target molecules for specific ligands. Anal. Biochem. 2015, 346, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Moraczewska, J.; Wawro, B.; Sugero, K.; Strzelecka-Gołaszewska, H. Divalent cation-, nucleotide-, and polymerization-dependent changes in the conformation of subdomain 2 of actin. Biophys. J. 1999, 77, 373–385. [VSports app下载 - Google Scholar] [CrossRef]
- Frazier, R.A.; Papadopoulou, A.; Green, R.J. Isothermal titration calorimetry study of epicatechin binding to serum albumin. J. Pharm. Biochem. Anal. 2006, 28, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Zaczyńska, D.; Pałecz, B.; Rachwał-Rosiak, D.; Belica, S.; den-Haan, H.; Pena-García, J.; Perez-Sanchez, H. Interactions of free and encapsulated hydroxycinnamic acids from green coffee with egg ovalbumin, whey and soy protein hydrolysates. LWT—Food Sci. Technol. 2016, 65, 823–831. [Google Scholar] [CrossRef]
- Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Bustamante-Rangel, M.; Carabias-Martínez, R. Pressurized liquid extraction as a sample preparation method for the analysis of isoflavones in pulses. Anal. Bioanal. Chem. 2012, 404, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yao, Y.; Zhu, Y.; Ren, G. Isoflavone content and composition in chickpea (Cicer arietinum L.) sprouts germinated under different conditions. J. Agric. Food Chem. 2015, 63, 2701–2707. [Google Scholar] [CrossRef] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aid. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [VSports在线直播 - Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the red clover sprouts are available from the authors. |


| Red Clover Sprouts * | Daidzein | Formononetin | Genistein | Biochanin A | Total |
|---|---|---|---|---|---|
| White light 10 | 40.14 ± 2.38 | 1449.87 ± 50.92 | 118.49 ± 4.88 | 212.08 ± 11.92 | 1820.58 ± 55.92 |
| UVA3 | 3.72 ± 0.17 | 721.37 ± 32.36 | 84.47 ± 6.39 | 192.84 ± 6.18 | 1002.40 ± 38.48 |
| UVA8 | 11.85 ± 0.36 | 794.36 ± 35.59 | 70.81 ± 3.18 | 152.10 ± 5.74 | 1348.32 ± 49.09 |
| UVB11 | 115.35 ± 7.39 | 496.15 ± 19.61 | 188.96 ± 6.96 | 353.15 ± 15.95 | 1153.61 ± 37.71 |
| Isoflavone/Red Clover Sprouts * | KA × 103 (L/mol) | ∆H (kJ/mol) | ∆S (J/mol·K) | ∆G (kJ/mol) | ∆Gpredicted (kJ/mol) |
|---|---|---|---|---|---|
| single isoflavones | |||||
| Daidzein | 1.34 ± 0.09 | −0.04 ± 0.01 | 57.85 ± 3.12 | −17.29 ± 0.71 | −35.98 |
| Formononetin | 2.41 ± 0.11 | 0.11 ± 0.02 | 62.99 ± 4.05 | −18.67 ± 0.90 b | −38.07 |
| Genistein | 0.82 ± 0.06 | 23.03 ± 1.38 a | 131.11 ± 10.92 | −16.08 ± 0.55 | −37.66 |
| Biochanin A | 2.87 ± 0.15 | 23.49 ± 1.08 a | 142.77 ± 9.15 | −19.09 ± 0.87 b | −38.49 |
| pairs of isoflavones | |||||
| Daidzein + Formononetin | - | −8.21 ± 0.60 | 36.03 ± 2.55 | −18.59 ± 0.92 a | - |
| Daidzein + Genistein | - | 0.28 ± 0.04 | 66.50 ± 5.96 | −18.88 ± 0.74 a | - |
| Daidzein + Biochanin A | - | −11.26 ± 0.71 | 15.55 ± 0.83 | −15.74 ± 0.39 | - |
| Formononetin + Genistein | - | 0.85 ± 0.07 | 60.94 ± 5.39 b | −16.71 ± 0.59 | - |
| Formononetin + Biochanin A | - | 5.11 ± 0.41 | 91.10 ± 7.50 | −21.14 ± 0.85 | - |
| Genistein + Biochanin A | - | 0.15 ± 0.01 | 62.98 ± 4.08 b | −18.00 ± 0.81 a | - |
| red clover sprouts extracts | |||||
| White light 10 | - | −22.57 ± 0.92 b | −35.89 ± 1.18 | −12.23 ± 0.43 | - |
| UVA3 | - | −22.19 ± 0.78 b | −9.01 ± 0.38 a | −19.59 ± 0.62 a | - |
| UVA8 | - | −21.19 ± 0.65 c | −5.23 ± 0.31 | −19.68 ± 0.71 a | - |
| UVB11 | - | −21.10 ± 1.05 c | −9.88 ± 0.43 a | −18.88 ± 0.65 a | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite (V体育安卓版)
Budryn, G.; Grzelczyk, J.; Pérez-Sánchez, H. Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells. Molecules 2018, 23, 2471. https://doi.org/10.3390/molecules23102471
Budryn G, Grzelczyk J, Pérez-Sánchez H. Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells. Molecules. 2018; 23(10):2471. https://doi.org/10.3390/molecules23102471
Chicago/Turabian StyleBudryn, Grażyna, Joanna Grzelczyk, and Horacio Pérez-Sánchez. 2018. "Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells" Molecules 23, no. 10: 2471. https://doi.org/10.3390/molecules23102471
APA StyleBudryn, G., Grzelczyk, J., & Pérez-Sánchez, H. (2018). Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells. Molecules, 23(10), 2471. https://doi.org/10.3390/molecules23102471