"VSports app下载" Elimination of Osteosarcoma by Necroptosis with Graphene Oxide-Associated Anti-HER2 Antibodies
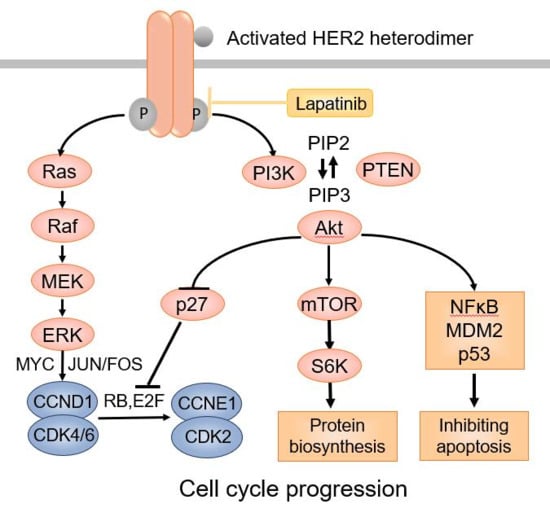
"> Figure 1
The HER2 signaling pathway.
"> Figure 2Mechanism of TRA/GO action. (A) TRA/GO induces ROS production. ROS disrupts mitochondrial integrity, releasing SMAC/DIBLO into cytosol. Binding of SAMC to cIAPs prevent RIP1 ubiquitylation, allowing formation of death complex ripoptosome. (B) Binding of TRA/GO to HER2 causes HER2 homodimerization, eliciting HER2 signaling; this activates NF-kB through the canonic pathway, augmenting TNFα production. TNFα singling through TNFR1 promotes degradation of cIAPs. (C) HER2 signaling also activates MAPK pathway, resulting in activation RSK2 that phosphorylate caspase 8 at Thr263 site to induce caspase ubiquitination and proteosomal degradation. Degradation of caspase 8 leads to formation of necroptosome executing necroptosis. (D) ROS also contributes to MAPK activation.
">
Abstract
The prognosis for non-resectable or recurrent osteosarcoma (OS) remains poor. The finding that the majority of OS overexpress the protooncogene HER2 raises the possibility of using HER2 as a therapeutic target. However, clinical trials on the anti-HER2 antibody trastuzumab (TRA) in treating OS find no therapeutic benefit. HER2 overexpression in OS is not generally associated with gene amplification, with low-level expression regarded as HER2 “negative”, as per criteria used to classify breast cancer HER2 status. Nevertheless, active HER2-targeting approaches, such as virus-based HER2 vaccines or CAR-T cells have generated promising results. More recently, it has been found that the noncovalent association of TRA with nanomaterial graphene oxide (GO) generates stable TRA/GO complexes capable of rapidly killing OS cells V体育官网入口. TRA/GO induces oxidative stress and strong HER2 signaling to elicit immediate degradation of both cIAP (cellular inhibitor of apoptosis protein) and caspase 8, leading to activation of necroptosis. This is an attractive mechanism of cancer cell death as chemo/apoptosis-resistant tumors may remain susceptible to necroptosis. In addition, necroptosis is potentially immunogenic to promote tumor immunity, as opposed to apoptosis that tends to silence tumor immunity. Currently, no established anticancer therapeutics are known to eliminate cancers by necroptosis. The aim of this article is to review the rationale and mechanisms of TRA/GO-mediated cytotoxicity. Keywords: osteosarcoma; necroptosis; HER2; graphene oxide; trastuzumab .1. Introduction
2. The Human Epidermal Growth Factor Receptor 2 (HER2) Expression in OS
3. Association of Trastuzumab (TRA) with Graphene Oxide (GO]
3.1. TRA Can Stably Associate with Graphene Oxide (GO) through Noncovalent Bonds
3.2. GO-Associated TRA Demonstrated Enhances Binding to HER2lo OS Cells
"VSports注册入口" 3.3. TRA/GO Is Cytotoxic to OS Cells
3.4. TRA/GO Induces Oxidative Stress as well as HER2 Signaling in OS Cells
3.5. TRA/GO Kills OS by Necroptosis (V体育官网)
3.6. TRA/GO Eliminate Established Xenograft OS
4. Discussion and Conclusions
Funding
Conflicts of Interest (VSports最新版本)
References
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. SICOT J. 2018, 4, 12. [V体育官网 - Google Scholar] [CrossRef]
- Ozaki, T.; Flege, S.; Liljenqvist, U.; Hillmann, A.; Delling, G.; Salzer-Kuntschik, M.; Jürgens, H.; Kotz, R.; Winkelmann, W.; Bielack, S.S. Osteosarcoma of the spine: Experience of the Cooperative Osteosarcoma Study Group. Cancer 2002, 94, 1069–1077. [Google Scholar] [CrossRef]
- O’Kane, G.M.; Cadoo, K.A.; Walsh, E.M.; Emerson, R.; Dervan, P.; O’Keane, C.; Hurson, B.; O’Toole, G.; Dudeney, S.; Kavanagh, E.; et al. Perioperative chemotherapy in the treatment of osteosarcoma: A 26-year single institution review. Clin. Sarcoma Res. 2015, 5, 17. [Google Scholar] [CrossRef]
- Bacci, G.; Ferrari, S.; Longhi, A.; Forni, C.; Bertoni, F.; Fabbri, N.; Zavatta, M.; Versari, M. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: Long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J. Chemother. 2001, 13, 93–99. [Google Scholar] [CrossRef]
- Schwartz, C.L.; Wexler, L.H.; Krailo, M.D.; Teot, L.A.; Devidas, M.; Steinherz, L.J.; Goorin, A.M.; Gebhardt, M.C.; Healey, J.H.; Sato, J.K.; et al. Intensified Chemotherapy With Dexrazoxane Cardioprotection in Newly Diagnosed Nonmetastatic Osteosarcoma: A Report From the Children’s Oncology Group. Pediatr. Blood Cancer 2016, 63, 54–61. [Google Scholar] [CrossRef]
- He, H.; Ni, J.; Huang, J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol. Lett. 2014, 7, 1352–1362. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar (V体育官网)] [CrossRef]
- Messerschmitt, P.J.; Rettew, A.N.; Brookover, R.E.; Garcia, R.M.; Getty, P.J.; Greenfield, E.M. Specific tyrosine kinase inhibitors regulate human osteosarcoma cells in vitro. Clin. Orthop. Relat. Res. 2008, 466, 2168–2175. [Google Scholar] [CrossRef][Green Version]
- Pommier, Y.; Sordet, O.; Antony, S.; Hayward, R.L.; Kohn, K.W. Apoptosis defects and chemotherapy resistance: Molecular interaction maps and networks. Oncogene 2004, 23, 2934–2949. [V体育2025版 - Google Scholar] [CrossRef]
- Chen, R.; Wang, G.; Zheng, Y.; Hua, Y.; Cai, Z. Drug resistance-related microRNAs in osteosarcoma: Translating basic evidence into therapeutic strategies. J. Cell Mol. Med. 2019, 23, 2280–2292. [Google Scholar] [CrossRef]
- Sekar, D.; Mani, P.; Biruntha, M.; Sivagurunathan, P.; Karthigeyan, M. Dissecting the functional role of microRNA 21 in osteosarcoma. Cancer Gene. Ther. 2019, 26, 179–182. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Li, B.; Wang, S.; Chen, T.; Ye, Z. Innate Immune Cells: A Potential and Promising Cell Population for Treating Osteosarcoma. Front. Immunol. 2019, 10, 1114. [Google Scholar (VSports app下载)] [CrossRef]
- Roberts, S.S.; Chou, A.J.; Cheung, N.K.V. Immunotherapy of Childhood Sarcomas. Front. Oncol. 2015, 5, 181. [VSports手机版 - Google Scholar] [CrossRef]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr. Blood Cancer 2018, 65, e27227. [Google Scholar] [CrossRef]
- Jimmy, R.; Stern, C.; Lisy, K.; White, S. Effectiveness of mifamurtide in addition to standard chemotherapy for high-grade osteosarcoma: A systematic review. JBI Database System. Rev. Implement. Rep. 2017, 15, 2113–2152. [Google Scholar (V体育安卓版)] [CrossRef]
- Yarden, Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 2001, 37, S3–S8. [Google Scholar (VSports手机版)] [CrossRef]
- Cho, H.S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W., Jr.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar (V体育平台登录)] [CrossRef]
- Brennan, P.J.; Kumogai, T.; Berezov, A.; Murali, R.; Greene, M.I. HER2/neu: Mechanisms of dimerization/oligomerization. Oncogene 2000, 19, 6093–6101. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Dori, S.; Vered, M.; David, R.; Buchner, A. HER2/neu expression in adenoid cystic carcinoma of salivary gland origin: An immunohistochemical study. J. Oral Pathol. Med. 2002, 31, 463–467. [Google Scholar] [CrossRef]
- Latif, Z.; Watters, A.D.; Bartlett, J.M.S.; Underwood, M.A.; Aitchison, M. Gene amplification and overexpression of HER2 in renal cell carcinoma. BJU Int. 2002, 89, 5–9. [Google Scholar (VSports app下载)] [CrossRef]
- Latif, Z.; Watters, A.D.; Dunn, I.; Grigor, K.; Underwood, M.A.; Bartlett, J.M.S. HER2/neu gene amplification and protein overexpression in G3 pT2 transitional cell carcinoma of the bladder: A role for anti-HER2 therapy? Eur. J. Cancer 2004, 40, 56–63. [Google Scholar (V体育2025版)] [CrossRef]
- Morris, M.J.; Reuter, V.E.; Kelly, W.K.; Slovin, S.F.; Kenneson, K.; Verbel, D.; Osman, I.; Scher, H.I. HER-2 profiling and targeting in prostate carcinoma. Cancer 2002, 94, 980–986. [Google Scholar] [CrossRef]
- Safran, H.; Steinhoff, M.; Mangray, S.; Rathore, R.; King, T.C.; Chai, L.; Berzein, K.; Moore, T.; Iannitti, D.; Reiss, P.; et al. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am. J. Clin. Oncol. 2001, 24, 496–499. ["VSports在线直播" Google Scholar] [CrossRef]
- Mar, N.; Vredenburgh, J.J.; Wasser, J.S. Targeting HER2 in the treatment of non-small cell lung cancer. Lung Cancer 2015, 87, 220–225. [VSports - Google Scholar] [CrossRef]
- Chavez-Blanco, A.; Perez-Sanchez, V.; Gonzalez-Fierro, A.; Vela-Chavez, T.; Candelaria, M.; Cetina, L.; Vidal, S.; Dueñas-Gonzalez, A. HER2 expression in cervical cancer as a potential therapeutic target. BMC Cancer 2004, 4, 59. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Morrison, C.; Zanagnolo, V.; Ramirez, N.; Cohn, D.E.; Kelbick, N.; Copeland, L.; Maxwell, L.G.; Fowler, J.M. HER-2 is an independent prognostic factor in endometrial cancer: Association with outcome in a large cohort of surgically staged patients. J. Clin. Oncol. 2006, 24, 2376–2385. [Google Scholar] [CrossRef]
- Oh, D.Y.; Kim, S.; Choi, Y.L.; Cho, Y.J.; Oh, E.; Choi, J.J.; Jung, K.; Song, J.Y.; Ahn, S.E.; Kim, B.G.; et al. HER2 as a novel therapeutic target for cervical cancer. Oncotarget 2015, 6, 36219–36230. [Google Scholar] [CrossRef]
- Pollock, N.I.; Grandis, J.R. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 526–533. [V体育官网 - Google Scholar] [CrossRef]
- Seigel, G.M.; Sharma, S.; Hackam, A.S.; Shah, D.K. HER2/ERBB2 immunoreactivity in human retinoblastoma. Tumour Biol. 2016, 37, 6135–6142. [Google Scholar] [CrossRef]
- Hegde, M.; Mukherjee, M.; Grada, Z.; Pignata, A.; Landi, D.; Navai, S.A.; Wakefield, A.; Fousek, K.; Bielamowicz, K.; Chow, K.K.; et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J. Clin. Investig. 2016, 126, 3036–3052. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. ["V体育ios版" Google Scholar] [CrossRef]
- Baselga, J. Herceptin alone or in combination with chemotherapy in the treatment of HER2-positive metastatic breast cancer: Pivotal trials. Oncology 2001, 61 (Suppl. 2), 14–21. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Hudis, C.A. Trastuzumab--mechanism of action and use in clinical practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef]
- Gorlick, R.; Huvos, A.G.; Heller, G.; Aledo, A.; Beardsley, G.P.; Healey, J.H.; Meyers, P.A. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J. Clin. Oncol. 1999, 17, 2781–2788. [Google Scholar] [CrossRef]
- Onda, M.; Matsuda, S.; Higaki, S.; Iijima, T.; Fukushima, J.I.; Yokokura, A.; Kojima, T.; Horiuchi, H.; Kurokawa, T.; Yamamoto, T. ErbB-2 expression is correlated with poor prognosis for patients with osteosarcoma. Cancer 1996, 77, 71–78. [Google Scholar] [CrossRef]
- Fellenberg, J.; Krauthoff, A.; Pollandt, K.; Delling, G.; Parsch, D. Evaluation of the predictive value of Her-2/neu gene expression on osteosarcoma therapy in laser-microdissected paraffin-embedded tissue. Lab. Investig. 2004, 84, 113–121. [VSports注册入口 - Google Scholar] [CrossRef]
- Thomas, D.G.; Giordano, T.J.; Sanders, D.; Biermann, J.S.; Baker, L. Absence of HER2/neu gene expression in osteosarcoma and skeletal Ewing’s sarcoma. Clin. Cancer Res. 2002, 8, 788–793. [Google Scholar]
- Willmore-Payne, C.; Holden, J.A.; Zhou, H.; Gupta, D.; Hirschowitz, S.; Wittwer, C.T.; Layfield, L.J. Evaluation of Her-2/neu gene status in osteosarcoma by fluorescence in situ hybridization and multiplex and monoplex polymerase chain reactions. Arch. Pathol. Lab. Med. 2006, 130, 691–698. [Google Scholar]
- Ahmed, N.; Salsman, V.S.; Yvon, E.; Louis, C.U.; Perlaky, L.; Wels, W.S.; Dishop, M.K.; Kleinerman, E.E.; Pule, M.; Rooney, C.M.; et al. Immunotherapy for osteosarcoma: Genetic modification of T cells overcomes low levels of tumor antigen expression. Mol. Ther. J. Am. Soc. Gene Ther. 2009, 17, 1779–1787. [Google Scholar] [CrossRef]
- Ebb, D.; Meyers, P.; Grier, H.; Bernstein, M.; Gorlick, R.; Lipshultz, S.E.; Krailo, M.; Devidas, M.; Barkauskas, D.A.; Siegal, G.P.; et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 2545–2551. [Google Scholar] [CrossRef]
- Mason, N.J.; Gnanandarajah, J.S.; Engiles, J.B.; Gray, F.; Laughlin, D.; Gaurnier-Hausser, A.; Wallecha, A.; Huebner, M.; Paterson, Y. Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin. Cancer Res. 2016, 22, 4380–4390. [Google Scholar] [CrossRef]
- Phillips, G.D.L.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Peters, S.; Stahel, R.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; Carpeno, J.D.C.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. ["VSports" Google Scholar] [CrossRef]
- Büchler, P.; Reber, H.A.; Bückler, M.C.; Roth, M.A.; Büchler, M.W.; Friess, H.; Isacoff, W.H.; Hines, O.J. Therapy for pancreatic cancer with a recombinant humanized anti-HER2 antibody (herceptin). J. Gastrointest. Surg. 2001, 5, 139–146. [Google Scholar] [CrossRef]
- Mihaljevic, A.; Büchler, P.; Harder, J.; Hofheinz, R.; Gregor, M.; Kanzler, S.; Schmiegel, W.; Heinemann, V.; Endlicher, E.; Klöppel, G.; et al. A prospective, non-randomized phase II trial of Trastuzumab and Capecitabine in patients with HER2 expressing metastasized pancreatic cancer. BMC Surg. 2009, 9, 1. [V体育安卓版 - Google Scholar] [CrossRef]
- Harder, J.; Ihorst, G.; Heinemann, V.; Hofheinz, R.; Moehler, M.; Buechler, P.; Kloeppel, G.; Röcken, C.; Bitzer, M.; Boeck, S.; et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br. J. Cancer 2012, 106, 1033–1038. [Google Scholar] [CrossRef]
- Li, L.; Luo, C.; Song, Z.; Reyes-Vargas, E.; Clayton, F.; Huang, J.; Jensen, P.; Chen, X. Association of anti-HER2 antibody with graphene oxide for curative treatment of osteosarcoma. Nanomedicine 2018, 14, 581–593. [Google Scholar] [CrossRef]
- Andreev, J.; Thambi, N.; Bay, A.E.P.; Delfino, F.; Martin, J.; Kelly, M.P.; Kirshner, J.R.; Rafique, A.; Kunz, A.; Nittoli, T.; et al. Bispecific Antibodies and Antibody-Drug Conjugates (ADCs) Bridging HER2 and Prolactin Receptor Improve Efficacy of HER2 ADCs. Mol. Cancer Ther. 2017, 16, 681–693. [Google Scholar] [CrossRef]
- Luo, C.; Deng, Z.; Li, L.; Clayton, F.; Chen, A.L.; Wei, R.; Miles, R.; Stephens, D.M.; Glenn, M.; Wang, X.; et al. Association of rituximab with graphene oxide confers direct cytotoxicity for CD20-positive lymphoma cells. Oncotarget 2016, 7, 12806–12822. [Google Scholar] [CrossRef]
- Li, H.; Fierens, K.; Zhang, Z.; Vanparijs, N.; Schuijs, M.J.; Van Steendam, K.; Feiner Gracia, N.; De Rycke, R.; De Beer, T.; De Beuckelaer, A.; et al. Spontaneous Protein Adsorption on Graphene Oxide Nanosheets Allowing Efficient Intracellular Vaccine Protein Delivery. ACS Appl. Mater. Interfaces 2016, 8, 1147–1155. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Shim, G.; Kim, M.G.; Park, J.Y.; Oh, Y.K. Graphene-based nanosheets for delivery of chemotherapeutics and biological drugs. Adv. Drug Deliv. Rev. 2016, 105, 205–227. [Google Scholar] [CrossRef]
- Yang, D.; Feng, L.; Dougherty, C.A.; Luker, K.E.; Chen, D.; Cauble, M.A.; Holl, M.M.B.; Luker, G.D.; Ross, B.D.; Liu, Z.; et al. In vivo targeting of metastatic breast cancer via tumor vasculature-specific nano-graphene oxide. Biomaterials 2016, 104, 361–371. [Google Scholar] [CrossRef]
- Taylor, R.B.; Duffus, W.P.H.; Raff, M.C.; Petris, S.D. Redistribution and pinocytosis of lymphocyte surface immunoglobulin molecules induced by anti-immunoglobulin antibody. Nat. N. Biol. 1971, 233, 225–229. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [VSports最新版本 - Google Scholar] [CrossRef]
- Listenberger, L.L.; Ory, D.S.; Schaffer, J.E. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 2001, 276, 14890–14895. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Chan, F.K.M.; Kroemer, G. Necroptosis: Mechanisms and Relevance to Disease. Annu. Rev. Pathol. 2017, 12, 103–130. [Google Scholar (V体育安卓版)] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar]
- Johnston, A.; Wang, Z. Necroptosis: MLKL Polymerization. J. Nat. Sci. 2018, 4, e513. [Google Scholar]
- Zhou, W.; Yuan, J. SnapShot: Necroptosis. Cell 2014, 158, 464. [Google Scholar] [CrossRef]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012, 150, 339–350. [Google Scholar (VSports注册入口)] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Ali, M.; Mocarski, E.S. Proteasome inhibition blocks necroptosis by attenuating death complex aggregation. Cell Death Dis. 2018, 9, 346. [Google Scholar (V体育平台登录)] [CrossRef]
- Esterberg, R.; Linbo, T.; Pickett, S.B.; Wu, P.; Ou, H.C.; Rubel, E.W.; Raible, D.W. Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J. Clin. Investig. 2016, 126, 3556–3566. [Google Scholar] [CrossRef]
- Vince, J.E.; Wong, W.W.L.; Khan, N.; Feltham, R.; Chau, D.; Ahmed, A.U.; Benetatos, C.A.; Chunduru, S.K.; Condon, S.M.; McKinlay, M.; et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 2007, 131, 682–693. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Blankenship, J.W.; Wayson, S.M.; Fedorova, A.V.; Kayagaki, N.; Garg, P.; Zobel, K.; Dynek, J.N.; Elliott, L.O.; Wallweber, H.J.; et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007, 131, 669–681. [Google Scholar] [CrossRef]
- Silke, J.; Rickard, J.A.; Gerlic, M. The diverse role of RIP kinases in necroptosis and inflammation. Nat. Immunol. 2015, 16, 689–697. ["V体育平台登录" Google Scholar] [CrossRef]
- Koike, A.; Hanatani, M.; Fujimori, K. Pan-caspase inhibitors induce necroptosis via ROS-mediated activation of mixed lineage kinase domain-like protein and p38 in classically activated macrophages. Exp. Cell Res. 2019, 380, 171–179. [V体育平台登录 - Google Scholar] [CrossRef]
- Shi, G.; Jia, P.; Chen, H.; Bao, L.; Feng, F.; Tang, H. Necroptosis occurs in osteoblasts during tumor necrosis factor-alpha stimulation and caspase-8 inhibition. Braz. J. Med. Biol. Res. 2018, 52, e7844. [Google Scholar] [CrossRef]
- Uluçkan, Ö.; Segaliny, A.; Botter, S.; Santiago, J.M.; Mutsaers, A.J. Preclinical mouse models of osteosarcoma. BoneKEy Rep. 2015, 4, 670. [Google Scholar] [CrossRef]
- Lenaz, G. Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta 1998, 1366, 53–67. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Vercesi, A.E. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. 1999, 26, 463–471. [Google Scholar] [CrossRef]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. ["V体育ios版" Google Scholar] [CrossRef]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [V体育平台登录 - Google Scholar] [CrossRef]
- Verhagen, A.M.; Ekert, P.G.; Pakusch, M.; Silke, J.; Connolly, L.M.; Reid, G.E.; Moritz, R.L.; Simpson, R.J.; Vaux, D.L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000, 102, 43–53. [Google Scholar] [CrossRef]
- Hannes, S.; Abhari, B.A.; Fulda, S. Smac mimetic triggers necroptosis in pancreatic carcinoma cells when caspase activation is blocked. Cancer Lett. 2016, 380, 31–38. [Google Scholar] [CrossRef]
- Collart, M.A.; Baeuerle, P.; Vassalli, P. Regulation of tumor necrosis factor alpha transcription in macrophages: Involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol. Cell. Biol. 1990, 10, 1498–1506. ["VSports" Google Scholar] [CrossRef]
- Kishore, R.; McMullen, M.R.; Cocuzzi, E.; Nagy, L.E. Lipopolysaccharide-mediated signal transduction: Stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp. Hepatol. 2004, 3, S31. ["V体育官网" Google Scholar] [CrossRef]
- Merkhofer, E.C.; Cogswell, P.; Baldwin, A.S. Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene 2010, 29, 1238–1248. [Google Scholar (V体育官网入口)] [CrossRef]
- Peng, C.; Cho, Y.Y.; Zhu, F.; Zhang, J.; Wen, W.; Xu, Y.; Yao, K.; Ma, W.Y.; Bode, A.M.; Dong, Z. Phosphorylation of caspase-8 (Thr-263) by ribosomal S6 kinase 2 (RSK2) mediates caspase-8 ubiquitination and stability. J. Biol. Chem. 2011, 286, 6946–6954. ["V体育安卓版" Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Kellert, B.; Dimitrova, D.P.; Langlais, C.; Hupe, M.; Cain, K.; MacFarlane, M.; Häcker, G.; Leverkus, M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 2011, 43, 449–463. [Google Scholar] [CrossRef]
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M.; et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002, 20, 719–726. [Google Scholar] [CrossRef]
- Fiorillo, M.; Verre, A.F.; Iliut, M.; Peiris-Pagés, M.; Ozsvari, B.; Gandara, R.; Cappello, A.R.; Sotgia, F.; Vijayaraghavan, A.; Lisanti, M.P. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget 2015, 6, 3553–3562. [Google Scholar] [CrossRef]
- Jin, G.; Liu, Y.; Xu, P. Induction of Necroptosis in Human Breast Cancer Drug-Resistant Cells by SMAC Analog LCL161 After Caspase Inhibition Requires RIP3. Pharmazie 2019, 74, 363–368. [Google Scholar]
- Su, Z.; Yang, Z.; Xie, L.; DeWitt, J.P.; Chen, Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016, 23, 748–756. [Google Scholar] [CrossRef]
- Najafov, A.; Chen, H.; Yuan, J. Necroptosis and Cancer. Trends Cancer 2017, 3, 294–301. ["V体育安卓版" Google Scholar] [CrossRef]
- Junttila, T.T.; Akita, R.W.; Parsons, K.; Fields, C.; Phillips, G.D.L.; Friedman, L.S.; Sampath, D.; Sliwkowski, M.X. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 2009, 15, 429–440. [Google Scholar] [CrossRef]
- Aaes, T.L.; Kaczmarek, A.; Delvaeye, T.; De Craene, B.; De Koker, S.; Heyndrickx, L.; Delrue, I.; Taminau, J.; Wiernicki, B.; De Groote, P.; et al. Vaccination with Necroptotic Cancer Cells Induces Efficient Anti-tumor Immunity. Cell Rep. 2016, 15, 274–287. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite (V体育安卓版)
Xiao, H.; Jensen, P.E.; Chen, X. Elimination of Osteosarcoma by Necroptosis with Graphene Oxide-Associated Anti-HER2 Antibodies. Int. J. Mol. Sci. 2019, 20, 4360. https://doi.org/10.3390/ijms20184360
Xiao H, Jensen PE, Chen X. Elimination of Osteosarcoma by Necroptosis with Graphene Oxide-Associated Anti-HER2 Antibodies. International Journal of Molecular Sciences. 2019; 20(18):4360. https://doi.org/10.3390/ijms20184360
Chicago/Turabian StyleXiao, Hongmei, Peter E. Jensen, and Xinjian Chen. 2019. "Elimination of Osteosarcoma by Necroptosis with Graphene Oxide-Associated Anti-HER2 Antibodies" International Journal of Molecular Sciences 20, no. 18: 4360. https://doi.org/10.3390/ijms20184360
APA StyleXiao, H., Jensen, P. E., & Chen, X. (2019). Elimination of Osteosarcoma by Necroptosis with Graphene Oxide-Associated Anti-HER2 Antibodies. International Journal of Molecular Sciences, 20(18), 4360. https://doi.org/10.3390/ijms20184360