"VSports" Belantamab Mafodotin with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma: A Comprehensive Exposure-Response Analysis of the DREAMM-8 Study
- PMID: 40958065
- PMCID: PMC12454471
- DOI: 10.1007/s11523-025-01174-0
Belantamab Mafodotin with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma: A Comprehensive Exposure-Response Analysis of the DREAMM-8 Study
Abstract
Background: In the phase 3 DREAMM-8 study (NCT04484623), belantamab mafodotin (anti-B-cell maturation antigen [BCMA] antibody-drug conjugate with a monomethyl auristatin F payload) with pomalidomide and dexamethasone (BPd) showed significant progression-free survival benefit in second-line or later relapsed/refractory multiple myeloma (RRMM). VSports手机版.
Objective: This exposure-response analysis explored the relationship between belantamab mafodotin cycle 1 exposure and efficacy/safety and predicted the benefit-risk profile of belantamab mafodotin at an initial dose of 1. 9 versus 2 V体育安卓版. 5 mg/kg using DREAMM-8 data. .
Patients and methods: In the BPd arm of DREAMM-8, belantamab mafodotin was dosed at 2 V体育ios版. 5 mg/kg intravenously in cycle 1, then at 1. 9 mg/kg every 4 weeks from cycle 2 onward. Cycle 1 belantamab mafodotin and free payload exposures derived from population pharmacokinetic analysis were used to perform exposure-efficacy/exposure-safety analyses for probability of/time to first event. Selected covariate effects were evaluated. .
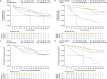
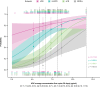
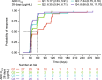
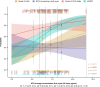
Results: Higher belantamab mafodotin cycle 1 exposure was associated with deeper response (higher probabilities of complete response or better [≥ CR] and minimal residual disease negativity), but not with grade ≥ 3 ocular adverse events (oAEs)/ophthalmic exam findings. Benefit-risk assessment showed that an initial belantamab mafodotin dose of 1. 9 mg/kg instead of 2 VSports最新版本. 5 mg/kg would result in reduction in probability of ≥ CR without reduction in oAEs/ophthalmic exam findings. .
Conclusions: An initial belantamab mafodotin dose of 2. 5 mg/kg for BPd yields deeper responses versus 1. 9 mg/kg with minimal change in safety outcomes in RRMM. DREAMM-8 (NCT04484623) was registered at clinicaltrials V体育平台登录. gov (21 July 2020). .
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Study 207499 and the analyses reported herein were funded by GSK. Drug linker technology licensed from Seagen Inc. ; monoclonal antibody produced using POTELLIGENT Technology licensed from BioWa. Conflicts of Interest: P. Hanafin, Y. L. Ho, T. Papathanasiou, G. Fulci, N. Sule, B. E. Kremer, and G. Ferron-Brady are employees of and hold financial equities in GSK. Ethics Approval: The DREAMM-8 trial protocol and amendments were approved by the appropriate ethics body at each participating institution VSports注册入口. Consent to Participate: All patients provided written informed consent before enrollment in DREAMM-8. Consent for Publication: N/A Data Availability: GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines, upon approval of proposals submitted to https://www. gsk-studyregister. com/en/ . Code Availability: N/A. Author Contributions: Conceptualization and design: P. Hanafin, Y. L. Ho, T. Papathanasiou, G. Fulci, N. Sule, B. E. Kremer, and G. Ferron-Brady; Data collection and analysis: P. Hanafin, Y. L. Ho, T. Papathanasiou, G. Fulci, N. Sule, B. E. Kremer, and G. Ferron-Brady; Writing—review and editing: P. Hanafin, Y. L. Ho, T. Papathanasiou, G. Fulci, N. Sule, B. E. Kremer, and G. Ferron-Brady. The first draft of the manuscript was written by Alexus Rivas-John and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Figures
References (V体育2025版)
-
- Dimopoulos MA, Hungria VTM, Radinoff A, Delimpasi S, Mikala G, Masszi T, et al. Efficacy and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3): a phase 3, open-label, randomised study. Lancet Haematol. 2023;10(10):e801–12. 10.1016/S2352-3026(23)00243-0. - PubMed
-
- Hungria V, Robak P, Hus M, Zherebtsova V, Ward C, Ho PJ, et al. Belantamab mafodotin, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2024;391(5):393–407. 10.1056/NEJMoa2405090. - PubMed
-
- Nooka AK, Cohen AD, Lee HC, Badros A, Suvannasankha A, Callander N, et al. Single-agent belantamab mafodotin in patients with relapsed/refractory multiple myeloma: final analysis of the DREAMM-2 trial. Cancer. 2023;129(23):3746–60. 10.1002/cncr.34987. - PMC (V体育ios版) - PubMed
Publication types
- VSports最新版本 - Actions
MeSH terms
- "VSports" Actions
- VSports手机版 - Actions
- "VSports在线直播" Actions
- Actions (VSports)
- Actions (V体育官网)
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- Actions (VSports最新版本)
Substances (VSports app下载)
- V体育平台登录 - Actions
- Actions (VSports最新版本)
"V体育平台登录" Associated data
- Actions (VSports在线直播)
"V体育官网入口" LinkOut - more resources
Full Text Sources
V体育平台登录 - Medical
Research Materials

