The role and mechanism of thrombospondin-4 in pulmonary arterial hypertension associated with congenital heart disease
- PMID: 39154161
- PMCID: PMC11330619
- DOI: 10.1186/s12931-024-02932-w
The role and mechanism of thrombospondin-4 in pulmonary arterial hypertension associated with congenital heart disease
Abstract
Background: Due to a special hemodynamic feature, pulmonary vascular disease in pulmonary arterial hypertension associated with congenital heart disease (PAH-CHD) has two stages: reversible and irreversible. So far, the mechanism involved in the transition from reversible to irreversible stage is elusive. Moreover, no recognized and reliable assessments to distinguish these two stages are available. Furthermore, we found that compared with control and reversible PAH, thrombospondin-4 (THBS4) was significantly upregulated in irreversible group by bioinformatic analysis VSports手机版. Hence, we further verify and investigate the expression and role of THBS4 in PAH-CHD. .
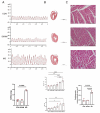
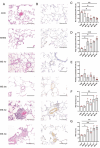
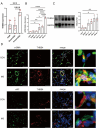
Methods: We established the monocrotaline plus aorto-cava shunt-induced (MCT-AV) rat model. We measured the expression of THBS4 in lung tissues from MCT-AV rats. Double immunofluorescence staining of lung tissue for THBS4 and α-SMA (biomarker of smooth muscle cells) or vWF (biomarker of endothelial cells) to identify the location of THBS4 in the pulmonary artery. Primary pulmonary artery smooth muscle cells (PASMCs) were cultivated, identified, and used in this study V体育安卓版. THBS4 was inhibited and overexpressed by siRNA and plasmid, respectively, to explore the effect of THBS4 on phenotype transformation, proliferation, apoptosis, and migration of PASMCs. The effect of THBS4 on pulmonary vascular remodeling was evaluated in vivo by adeno-associated virus which suppressed THBS4 expression. Circulating level of THBS4 in patients with PAH-CHD was measured by ELISA. .
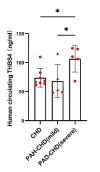
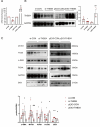
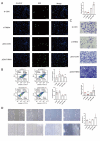
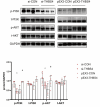
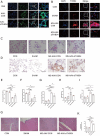
Results: THBS4 was upregulated in the lung tissues of MCT-AV rats, and was further upregulated in severe pulmonary vascular lesions. And THBS4 was expressed mainly in PASMCs V体育ios版. When THBS4 was inhibited, contractile markers α-SMA and MYH11 were upregulated, while the proliferative marker PCNA was decreased, the endothelial-mensenchymal transition marker N-cad was downregulated, proapototic marker BAX was increased. Additionally, proliferation and migration of PASMCs was inhibited and apoptosis was increased. Conversely, THBS4 overexpression resulted in opposite effects. And the impact of THBS4 on PASMCs was probably achieved through the regulation of the PI3K/AKT pathway. THBS4 suppression attenuated pulmonary vascular remodeling. Furthermore, compared with patients with simple congenital heart disease and mild PAH-CHD, the circulating level of THBS4 was higher in patients with severe PAH-CHD. .
Conclusions: THBS4 is a promising biomarker to distinguish reversible from irreversible PAH-CHD before repairing the shunt. THBS4 is a potential treatment target in PAH-CHD, especially in irreversible stage. VSports最新版本.
Keywords: Congenital heart disease; Pulmonary arterial hypertension; Pulmonary vascular remodeling; Reversibility; Thrombospondin-4. V体育平台登录.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- van Riel AC, Schuuring MJ, van Hessen ID, Zwinderman AH, Cozijnsen L, Reichert CL, Hoorntje JC, Wagenaar LJ, Post MC, van Dijk AP, et al. Contemporary prevalence of pulmonary arterial hypertension in adult congenital heart disease following the updated clinical classification. Int J Cardiol. 2014;174:299–305. 10.1016/j.ijcard.2014.04.072 - DOI - PubMed
-
- Lammers AE, Bauer LJ, Diller GP, Helm PC, Abdul-Khaliq H, Bauer UMM, Baumgartner H. Pulmonary hypertension after shunt closure in patients with simple congenital heart defects. Int J Cardiol. 2020;308:28–32. 10.1016/j.ijcard.2019.12.070 - V体育官网入口 - DOI - PubMed
-
- van Loon RL, Roofthooft MT, Hillege HL, ten Harkel AD, van Osch-Gevers M, Delhaas T, Kapusta L, Strengers JL, Rammeloo L, Clur SA, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755–64. 10.1161/CIRCULATIONAHA.110.969584 - DOI - PubMed
MeSH terms (V体育安卓版)
- "V体育官网入口" Actions
- "V体育安卓版" Actions
- VSports - Actions
- VSports注册入口 - Actions
- "V体育平台登录" Actions
- Actions (VSports app下载)
- V体育2025版 - Actions
- Actions (V体育平台登录)
- VSports app下载 - Actions
- "V体育2025版" Actions
- "V体育官网" Actions
VSports app下载 - Substances
- VSports app下载 - Actions
- VSports手机版 - Actions
LinkOut - more resources
Full Text Sources
Medical
VSports app下载 - Research Materials
"VSports app下载" Miscellaneous

