VSports手机版 - Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study
- PMID: 35617989
- PMCID: PMC9126559
- DOI: 10.1016/S1470-2045(22)00202-9
"VSports最新版本" Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study
Abstract (V体育官网)
Background: People with cancer are at increased risk of hospitalisation and death following infection with SARS-CoV-2 VSports手机版. Therefore, we aimed to conduct one of the first evaluations of vaccine effectiveness against breakthrough SARS-CoV-2 infections in patients with cancer at a population level. .
Methods: In this population-based test-negative case-control study of the UK Coronavirus Cancer Evaluation Project (UKCCEP), we extracted data from the UKCCEP registry on all SARS-CoV-2 PCR test results (from the Second Generation Surveillance System), vaccination records (from the National Immunisation Management Service), patient demographics, and cancer records from England, UK, from Dec 8, 2020, to Oct 15, 2021. Adults (aged ≥18 years) with cancer in the UKCCEP registry were identified via Public Health England's Rapid Cancer Registration Dataset between Jan 1, 2018, and April 30, 2021, and comprised the cancer cohort. We constructed a control population cohort from adults with PCR tests in the UKCCEP registry who were not contained within the Rapid Cancer Registration Dataset. The coprimary endpoints were overall vaccine effectiveness against breakthrough infections after the second dose (positive PCR COVID-19 test) and vaccine effectiveness against breakthrough infections at 3-6 months after the second dose in the cancer cohort and control population. V体育安卓版.
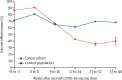
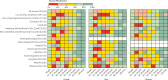
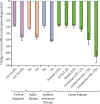
Findings: The cancer cohort comprised 377 194 individuals, of whom 42 882 had breakthrough SARS-CoV-2 infections. The control population consisted of 28 010 955 individuals, of whom 5 748 708 had SARS-CoV-2 breakthrough infections. Overall vaccine effectiveness was 69·8% (95% CI 69·8-69·9) in the control population and 65·5% (65·1-65·9) in the cancer cohort V体育ios版. Vaccine effectiveness at 3-6 months was lower in the cancer cohort (47·0%, 46·3-47·6) than in the control population (61·4%, 61·4-61·5). .
Interpretation: COVID-19 vaccination is effective for individuals with cancer, conferring varying levels of protection against breakthrough infections VSports最新版本. However, vaccine effectiveness is lower in patients with cancer than in the general population. COVID-19 vaccination for patients with cancer should be used in conjunction with non-pharmacological strategies and community-based antiviral treatment programmes to reduce the risk that COVID-19 poses to patients with cancer. .
Funding: University of Oxford, University of Southampton, University of Birmingham, Department of Health and Social Care, and Blood Cancer UK V体育平台登录. .
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4. 0 license. Published by Elsevier Ltd VSports注册入口. All rights reserved. .
Conflict of interest statement (VSports最新版本)
Declaration of interests We declare no competing interests.
Figures

Comment in
-
V体育官网入口 - COVID-19 vaccine effectiveness in patients with cancer: remaining vulnerabilities and uncertainties.Lancet Oncol. 2022 Jun;23(6):693-695. doi: 10.1016/S1470-2045(22)00252-2. Epub 2022 May 23. Lancet Oncol. 2022. PMID: 35617990 Free PMC article. No abstract available.
References (VSports手机版)
-
- Lee LYW, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. - "VSports在线直播" PMC - PubMed
-
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. - V体育平台登录 - PMC - PubMed
Publication types
- Actions (VSports最新版本)
MeSH terms
- Actions (V体育ios版)
- Actions (VSports注册入口)
- VSports在线直播 - Actions
- VSports最新版本 - Actions
Substances
- "V体育平台登录" Actions
Supplementary concepts
"V体育官网入口" Grants and funding
LinkOut - more resources (V体育平台登录)
Full Text Sources
Medical
"VSports" Research Materials
Miscellaneous

