Predicting high-grade prostate cancer at initial biopsy: clinical performance of the ExoDx (EPI) Prostate Intelliscore test in three independent prospective studies (VSports在线直播)
- PMID: 34593984
- PMCID: PMC9184274
- DOI: 10.1038/s41391-021-00456-8
VSports最新版本 - Predicting high-grade prostate cancer at initial biopsy: clinical performance of the ExoDx (EPI) Prostate Intelliscore test in three independent prospective studies
Abstract
Background: The ability to discriminate indolent from clinically significant prostate cancer (PC) at the initial biopsy remains a challenge VSports手机版. The ExoDx Prostate (IntelliScore) (EPI) test is a noninvasive liquid biopsy that quantifies three RNA targets in urine exosomes. The EPI test stratifies patients for risk of high-grade prostate cancer (HGPC; ≥ Grade Group 2 [GG] PC) in men ≥ 50 years with equivocal prostate-specific antigen (PSA) (2-10 ng/mL). Here, we present a pooled meta-analysis from three independent prospective-validation studies in men presenting for initial biopsy decision. .
Methods: Pooled data from two prospective multi-site validation studies and the control arm of a clinical utility study were analyzed. Performance was evaluated using the area under the receiver-operating characteristic curve (AUC), negative predictive value (NPV), positive predictive value (PPV), sensitivity, and specificity for discriminating ≥ GG2 from GG1 and benign pathology. V体育安卓版.
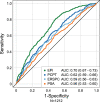
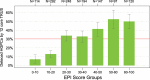
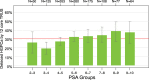
Results: The combined cohort (n = 1212) of initial-biopsy subjects had a median age of 63 years and median PSA of 5. 2 ng/mL. The EPI AUC (0. 70) was superior to PSA (0. 56), Prostate Cancer Prevention Trial Risk Calculator (PCPT-RC) (0. 62), and The European Randomized Study of Screening for Prostate Cancer (ERSPC) (0. 59), (all p-values <0. 001) for discriminating GG2 from GG1 and benign histology V体育ios版. The validated cutoff of 15. 6 would avoid 23% of all prostate biopsies and 30% of "unnecessary" (benign or Gleason 6/GG1) biopsies, with an NPV of 90%. .
Conclusions: EPI is a noninvasive, easy-to-use, urine exosome-RNA assay that has been validated across 3 independent prospective multicenter clinical trials with 1212 subjects. The test can discriminate high-grade (≥GG2) from low-grade (GG1) cancer and benign disease. EPI effectively guides the biopsy-decision process independent of PSA and other standard-of-care factors VSports最新版本. .
© 2021. The Author(s).
Conflict of interest statement
P Torkler, M Noerholm, V Tadigotla, C Fischer, and J Skog are employees of Exosome Diagnostics, a Bio-Techne Brand; M Donovan is a consultant to Exosome Diagnostics.
"VSports" Figures

References
Publication types
- "VSports手机版" Actions
MeSH terms
- "V体育安卓版" Actions
- "V体育官网" Actions
- Actions (V体育官网入口)
- "V体育安卓版" Actions
- VSports - Actions
- V体育安卓版 - Actions
- Actions (VSports在线直播)
Substances
- Actions (V体育官网入口)
- V体育官网 - Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials (VSports手机版)
Miscellaneous

