VDAC1 Silencing in Cancer Cells Leads to Metabolic Reprogramming That Modulates Tumor Microenvironment
- PMID: 34200480
- PMCID: PMC8201394
- DOI: 10.3390/cancers13112850
VDAC1 Silencing in Cancer Cells Leads to Metabolic Reprogramming That Modulates Tumor Microenvironment (V体育平台登录)
Abstract
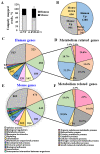
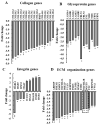
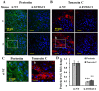
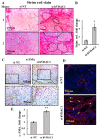
The tumor microenvironment (TME) plays an important role in cell growth, proliferation, migration, immunity, malignant transformation, and apoptosis. Thus, better insight into tumor-host interactions is required VSports手机版. Most of these processes involve the metabolic reprogramming of cells. Here, we focused on this reprogramming in cancerous cells and its effect on the TME. A major limitation in the study of tumor-host interactions is the difficulty in separating cancerous from non-cancerous signaling pathways within a tumor. Our strategy involved specifically silencing the expression of VDAC1 in the mitochondria of human-derived A549 lung cancer xenografts in mice, but not in the mouse-derived cells of the TME. Next-generation sequencing (NGS) analysis allows distinguishing the human or mouse origin of genes, thus enabling the separation of the bidirectional cross-talk between the TME and malignant cells. We demonstrate that depleting VDAC1 in cancer cells led to metabolic reprogramming, tumor regression, and the disruption of tumor-host interactions. This was reflected in the altered expression of a battery of genes associated with TME, including those involved in extracellular matrix organization and structure, matrix-related peptidases, angiogenesis, intercellular interacting proteins, integrins, and growth factors associated with stromal activities. We show that metabolic rewiring upon mitochondrial VDAC1 silencing in cancer cells affected several components of the TME, such as structural protein matrix metalloproteinases and Lox, and elicited a stromal response resembling the reaction to a foreign body in wound healing. As tumor progression requires a cooperative interplay between the host and cancer cells, and the ECM is intensively remodeled during cancer progression, VDAC1 depletion induced metabolic reprogramming that targeted both tumor cells and resulted in the alteration of the whole spectrum of TME-related genes, affecting the reciprocal feedback between ECM molecules, host cells, and cancer cells. Thus, VDAC1 depletion using si-VDAC1 represents therapeutic potential, inhibiting cancer cell proliferation and also inducing the modulation of TME components, which influences cancer progression, migration, and invasion. .
Keywords: VDAC1; metabolism; mitochondria; reprogramming; siRNA; tumor microenvironment V体育安卓版. .
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References (V体育2025版)
-
- Cantor J.R., Sabatini D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD (V体育2025版)-12-0345. - DOI - PMC - PubMed
"VSports最新版本" LinkOut - more resources
Full Text Sources (V体育安卓版)
Research Materials

