"V体育官网" Defining Treatment-Related Adverse Effects in Patients with Glioma: Distinctive Features of Pseudoprogression and Treatment-Induced Necrosis
- PMID: 32488924
- PMCID: PMC7418360
- DOI: 10.1634/theoncologist.2020-0085
Defining Treatment-Related Adverse Effects in Patients with Glioma: Distinctive Features of Pseudoprogression and Treatment-Induced Necrosis
"VSports在线直播" Abstract
Background: Pseudoprogression (PP) and treatment-induced brain tissue necrosis (TN) are challenging cancer treatment-related effects. Both phenomena remain insufficiently defined; differentiation from recurrent disease frequently necessitates tissue biopsy. We here characterize distinctive features of PP and TN to facilitate noninvasive diagnosis and clinical management. VSports手机版.
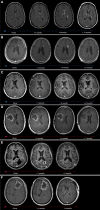
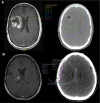
Materials and methods: Patients with glioma and confirmed PP (defined as appearance <5 months after radiotherapy [RT] completion) or TN (>5 months after RT) were retrospectively compared using clinical, radiographic, and histopathological data V体育安卓版. Each imaging event/lesion (region of interest [ROI]) diagnosed as PP or TN was longitudinally evaluated by serial imaging. .
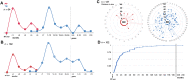
Results: We identified 64 cases of mostly (80%) biopsy-confirmed PP (n = 27) and TN (n = 37), comprising 137 ROIs in total V体育ios版. Median time of onset for PP and TN was 1 and 11 months after RT, respectively. Clinically, PP occurred more frequently during active antineoplastic treatment, necessitated more steroid-based interventions, and was associated with glioblastoma (81 vs. 40%), fewer IDH1 mutations, and shorter median overall survival. Radiographically, TN lesions often initially manifested periventricularly (n = 22/37; 60%), were more numerous (median, 2 vs. 1 ROIs), and contained fewer malignant elements upon biopsy. By contrast, PP predominantly developed around the tumor resection cavity as a non-nodular, ring-like enhancing structure. Both PP and TN lesions almost exclusively developed in the main prior radiation field. Presence of either condition appeared to be associated with above-average overall survival. .
Conclusion: PP and TN occur in clinically distinct patient populations and exhibit differences in spatial radiographic pattern. Increased familiarity with both conditions and their unique features will improve patient management and may avoid unnecessary surgical procedures. VSports最新版本.
Implications for practice: Pseudoprogression (PP) and treatment-induced brain tissue necrosis (TN) are challenging treatment-related effects mimicking tumor progression in patients with brain cancer. Affected patients frequently require surgery to guide management V体育平台登录. PP and TN remain arbitrarily defined and insufficiently characterized. Lack of clear diagnostic criteria compromises treatment and may adversely affect outcome interpretation in clinical trials. The present findings in a cohort of patients with glioma with PP/TN suggest that both phenomena exhibit unique clinical and imaging characteristics, manifest in different patient populations, and should be classified as distinct clinical conditions. Increased familiarity with PP and TN key features may guide clinicians toward timely noninvasive diagnosis, circumvent potentially unnecessary surgical procedures, and improve response assessment in neuro-oncology. .
Keywords: Malignant glioma; Neurotoxicity; Pseudoprogression; Tissue necrosis; Treatment-related effects. VSports注册入口.
© 2020 The Authors V体育官网入口. The Oncologist published by Wiley Periodicals LLC on behalf of AlphaMed Press. .
VSports在线直播 - Conflict of interest statement
Figures

References
-
- Dietrich J, Winter S, Klein J. Neuroimaging of brain tumors: Pseudoprogression, pseudores‐ponse, and delayed effects of chemotherapy and radiation. Semin Neurol 2017;37:589–596. - PubMed
-
- Karschnia P, Parsons MW, Dietrich J. Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet Oncol 2019;20:e92–e102. - PubMed
-
- Brandsma D, Stalpers L, Taal W et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008;9:453–461. - PubMed
-
- Eisele SC, Dietrich J. Cerebral radiation necrosis: Diagnostic challenge and clinical management. Rev Neurol 2015;61:225–232. - PubMed
MeSH terms
- "VSports最新版本" Actions
- V体育官网 - Actions
- "V体育安卓版" Actions
- Actions (V体育官网入口)
- "VSports手机版" Actions
LinkOut - more resources
Full Text Sources
Medical
"V体育官网" Miscellaneous

