Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers
- PMID: 31899616
- PMCID: PMC7180072
- DOI: 10.1021/acschembio.9b00939
Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers
Abstract
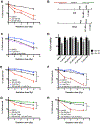
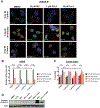
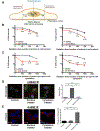
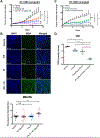
Although radiation is widely used to treat cancers, resistance mechanisms often develop and involve activation of DNA repair and inhibition of apoptosis. Therefore, compounds that sensitize cancer cells to radiation via alternative cell death pathways are valuable. We report here that ferroptosis, a form of nonapoptotic cell death driven by lipid peroxidation, is partly responsible for radiation-induced cancer cell death. Moreover, we found that small molecules activating ferroptosis through system xc- inhibition or GPX4 inhibition synergize with radiation to induce ferroptosis in several cancer types by enhancing cytoplasmic lipid peroxidation but not increasing DNA damage or caspase activation. Ferroptosis inducers synergized with cytoplasmic irradiation, but not nuclear irradiation. Finally, administration of ferroptosis inducers enhanced the antitumor effect of radiation in a murine xenograft model and in human patient-derived models of lung adenocarcinoma and glioma. These results suggest that ferroptosis inducers may be effective radiosensitizers that can expand the efficacy and range of indications for radiation therapy VSports手机版. .
Conflict of interest statement
CONFLICT OF INTERESTS
B V体育安卓版. R. S. holds equity in and serves as a consultant to Inzen Therapeutics.
Figures



References
-
- Delaney G, Jacob S, Featherstone C, and Barton M (2005) The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines, Cancer 104, 1129–1137. - PubMed
-
- Souhami RL, and Tobias JS (2003) Cancer and its management, 4th ed., Blackwell Science, Malden, Mass.
-
- Gerszten PC, Mendel E, and Yamada Y (2009) Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes?, Spine (Phila Pa 1976) 34, S78–92. - PubMed
Publication types
- "VSports app下载" Actions
"V体育2025版" MeSH terms
- Actions (V体育官网入口)
- VSports在线直播 - Actions
- Actions (V体育官网)
- Actions (VSports)
- V体育官网 - Actions
- V体育安卓版 - Actions
Substances
- V体育官网入口 - Actions
- "VSports app下载" Actions
- Actions (V体育官网)
- "V体育平台登录" Actions
- "VSports" Actions
- "V体育2025版" Actions
V体育官网入口 - Grants and funding
LinkOut - more resources (VSports在线直播)
Full Text Sources
Other Literature Sources
Medical (VSports在线直播)

