VSports手机版 - A Novel ALDH1A1 Inhibitor Targets Cells with Stem Cell Characteristics in Ovarian Cancer
- PMID: 30965686
- PMCID: PMC6521036 (VSports最新版本)
- DOI: 10.3390/cancers11040502
A Novel ALDH1A1 Inhibitor Targets Cells with Stem Cell Characteristics in Ovarian Cancer
Abstract
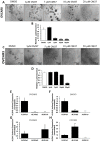
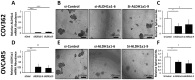
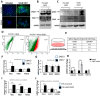
A small of population of slow cycling and chemo-resistant cells referred to as cancer stem cells (CSC) have been implicated in cancer recurrence. There is emerging interest in developing targeted therapeutics to eradicate CSCs. Aldehyde-dehydrogenase (ALDH) activity was shown to be a functional marker of CSCs in ovarian cancer (OC). ALDH activity is increased in cells grown as spheres versus monolayer cultures under differentiating conditions and in OC cells after treatment with platinum. Here, we describe the activity of CM37, a newly identified small molecule with inhibitory activity against ALDH1A1, in OC models enriched in CSCs. Treatment with CM37 reduced OC cells' proliferation as spheroids under low attachment growth conditions and the expression of stemness-associated markers (OCT4 and SOX2) in ALDH+ cells fluorescence-activated cell sorting (FACS)-sorted from cell lines and malignant OC ascites. Likewise, siRNA-mediated ALDH1A1 knockdown reduced OC cells' proliferation as spheres, expression of stemness markers, and delayed tumor initiation capacity in vivo. Treatment with CM37 promoted DNA damage in OC cells, as evidenced by induction of γH2AX. This corresponded to increased expression of genes involved in DNA damage response, such as NEIL3, as measured in ALDH+ cells treated with CM37 or in cells where ALDH1A1 was knocked down. By inhibiting ALDH1A1, CM37 augmented intracellular ROS accumulation, which in turn led to increased DNA damage and reduced OC cell viability. Cumulatively, our findings demonstrate that a novel ALDH1A1 small molecule inhibitor is active in OC models enriched in CSCs. Further optimization of this new class of small molecules could provide a novel strategy for targeting treatment-resistant OC. VSports手机版.
Keywords: ALDH1A1; CM37; cancer stem cells; ovarian cancer V体育安卓版. .
"V体育2025版" Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. - V体育2025版 - DOI - PubMed
-
- Silva I.A., Bai S., McLean K., Yang K., Griffith K., Thomas D., Ginestier C., Johnston C., Kueck A., Reynolds R.K., et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. - DOI - PMC - PubMed
-
- Zhang S., Balch C., Chan M.W., Lai H.C., Matei D., Schilder J.M., Yan P.S., Huang T.H., Nephew K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. - "VSports" DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
VSports在线直播 - Full Text Sources
Miscellaneous

