Do patients undergoing cytoreductive surgery and HIPEC for peritoneal malignancy need parenteral nutrition?
- PMID: 30911667
- PMCID: PMC6404997
- DOI: V体育2025版 - 10.1515/pp-2018-0123
VSports - Do patients undergoing cytoreductive surgery and HIPEC for peritoneal malignancy need parenteral nutrition?
"VSports手机版" Abstract
Background: To analyse the duration of parenteral nutrition (PN) in patients treated for peritoneal malignancy with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) over a 2 year period at a single UK National referral centre VSports手机版. .
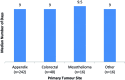
Methods: A retrospective analysis of prospective data for all patients (n=321) who underwent CRS and HIPEC for peritoneal malignancy at the Peritoneal Malignancy Institute Basingstoke between April 1, 2013 and March 31, 2015. Duration of PN was compared between primary tumour site (appendix, colorectal, mesothelioma and other); completeness of CRS (complete CRS vs. major tumour debulking) and pre-operative nutritional assessment measures (including Mid Upper Arm Circumference) V体育安卓版. .
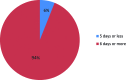
Results: The median duration of PN was 9 days (range 2-87 days). A total of 13 % of patients had PN for less than 7 days and 6 % for 5 days or less. There was no significant difference in duration of PN between the different tumour sites. Two factors that may increase the duration of PN include having major tumour debulking (MTD) and a baseline MUAC<23. 5 cm V体育ios版. .
Conclusions: Most patients who underwent CRS and HIPEC for peritoneal malignancy required PN for more than 7 days with poor pre-operative nutritional status and inability to achieve complete cytoreduction predictors of prolonged PN requirements VSports最新版本. .
Keywords: Pseudomyxoma peritonei; peritoneal surface malignancies; total parenteral nutrition V体育平台登录. .
Conflict of interest statement
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Figures
References
-
- Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–31. - PubMed
-
- Youssef H, Newman C, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Operative findings, early complications and long-term survival in 456 patients with pseudomyxoma peritonei of appendiceal origin. Dis Colon Rectum. 2011;54:293–9. - PubMed
-
- Moran BJ, Cecil TD. The aetiology, clinical presentation, and management of pseudomyxoma peritonei. Sug Oncol Clin North Am. 2003;12:385–603. - PubMed
-
- Yan TD, Deraco M, Baratti D, Kusamuna S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–42. - PubMed
-
- Chua TC, Yan TD, Deraco M, Glehen O, Moran BJ, Sugarbaker PH. Peritoneal Surface Oncology Group. Multi-institutional experience of diffuse intra-abdominal multicystic peritoneal mesothelioma. Br J Surg. 2011;98:60–4. - VSports app下载 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous