VSports手机版 - Down-regulation of OGT promotes cisplatin resistance by inducing autophagy in ovarian cancer
- PMID: 30555541
- PMCID: V体育ios版 - PMC6276088
- DOI: "VSports手机版" 10.7150/thno.27806
Down-regulation of OGT promotes cisplatin resistance by inducing autophagy in ovarian cancer
Abstract
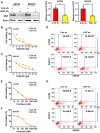
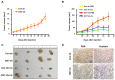
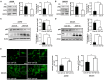
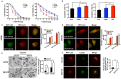
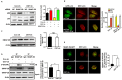
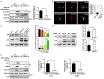
Cisplatin resistance significantly affects the survival rate of patients with ovarian cancer. However, the main mechanism underlying cisplatin resistance in ovarian cancer remains unclear. Methods: Immunohistochemistry was used to determine the expression of OGT, OGA and O-GlcNAc in chemoresistant and chemosensitive ovarian cancer tissues VSports手机版. Functional analyses (in vitro and in vivo) were performed to confirm the role of OGT in cisplatin resistance. Autophagy-related proteins were tested by western blot. Transmission electron microscopy and mRFP-GFP-LC3 adenovirus reporter were used for autophagy flux analysis. Immunoprecipitation assay was utilized to detect protein-protein interactions. Results: We found that O-GlcNAc and O-GlcNAc transferase (OGT) levels were significantly lower in chemoresistant ovarian cancer tissues than in chemosensitive tissues, whereas O-GlcNAcase (OGA) levels did not differ. The down-regulation of OGT increased cisplatin resistance in ovarian cancer cells but had no effect on the efficacy of paclitaxel. The down-regulation of OGT improved tumor resistance to cisplatin in a mouse xenograft tumor model. OGT knockdown enhanced cisplatin-induced autophagy, which reduced apoptotic cell death induced by cisplatin, and promoted autolysosome formation. A reduction in O-GlcNAcylated SNAP-29 levels caused by the down-regulation of OGT promoted the formation of the SNARE complex and autophagic flux. Conclusion: Our findings suggest that down-regulation of OGT enhances cisplatin-induced autophagy via SNAP-29, resulting in cisplatin-resistant ovarian cancer. OGT may represent a novel target for overcoming cisplatin resistance in ovarian cancer. .
Keywords: OGT; SNARE V体育安卓版. ; autophagy; chemoresistance; ovarian cancer. .
V体育平台登录 - Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Chornokur G, Amankwah EK, Schildkraut JM. et al. Global ovarian cancer health disparities. J Gynecol Oncol. 2013;129:258–64. - PMC (VSports在线直播) - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Kobayashi H, Yamada Y, Sado T. et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int J Gynecol Cancer. 2008;18:414–20. - PubMed
-
- Buys SS, Partridge E, Black A. et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–303. - PubMed
Publication types
MeSH terms
- VSports - Actions
- "VSports app下载" Actions
- Actions (V体育2025版)
- V体育平台登录 - Actions
- "V体育2025版" Actions
- Actions (V体育安卓版)
- VSports注册入口 - Actions
- Actions (V体育官网入口)
- V体育平台登录 - Actions
- Actions (V体育平台登录)
- V体育官网 - Actions
- V体育官网 - Actions
- "V体育ios版" Actions
Substances
- V体育2025版 - Actions
- V体育ios版 - Actions
- V体育ios版 - Actions
- Actions (VSports)
LinkOut - more resources
Full Text Sources
Medical (VSports手机版)
Molecular Biology Databases
Miscellaneous (V体育2025版)

