"VSports在线直播" Morin decreases galectin-3 expression and sensitizes ovarian cancer cells to cisplatin
- PMID: 30267152
- PMCID: PMC6244704
- DOI: 10.1007/s00404-018-4912-4
Morin decreases galectin-3 expression and sensitizes ovarian cancer cells to cisplatin
V体育2025版 - Abstract
Purpose: This study aimed at evaluating whether morin (a natural flavonoid and a known inhibitor of NF-κB) can sensitize ovarian cancer cells to cisplatin by decreasing the expression of galectin-3, which is an anti-apoptotic protein regulated by NF-κB transcription factor VSports手机版. .
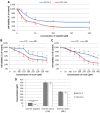
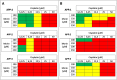
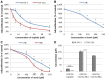
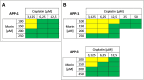
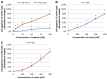
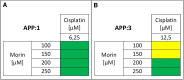
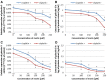
Methods: To assess the possibility of augmentation the activity of cisplatin by morin, we studied the separate and the combined effect of morin and cisplatin on viability, proliferation, and apoptosis of TOV-21G (cisplatin-sensitive) and SK-OV-3 (cisplatin-resistant) ovarian cancer cells V体育安卓版. We also analysed the effect of morin and cisplatin on galectin-3 expression at the mRNA and protein levels. .
Results: We demonstrated that morin possess antitumor activity against TOV-21G and SK-OV-3 ovarian cancer cells by reducing cell viability and proliferation as well as increasing the induction of apoptosis. Co-treatment of the cells with selected concentrations of morin and cisplatin, accordingly to specific treatment approaches, reveals a synergism, which leads to sensitization of the cells to cisplatin. During this sensitization, morin significantly reduces the expression of galectin-3 at the mRNA and protein level, regardless of the presence of cisplatin. V体育ios版.
Conclusions: Morin sensitizes TOV-21G and SK-OV-3 ovarian cancer cells to cisplatin, what is associated with a decrease of the expression of galectin-3 VSports最新版本. .
Keywords: Cisplatin; Drug resistance; Galectin-3; Morin; Ovarian cancer. V体育平台登录.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures
References
-
- Arzuman L, Beale P, Chan C, et al. Synergism from combinations of tris (benzimidazole) monochloroplatinum(II) chloride with capsaicin, quercetin, curcumin and cisplatin in human ovarian cancer cell lines. Anticancer Res. 2014;34:5453–5464. - PubMed
Publication types
- V体育安卓版 - Actions
MeSH terms
- VSports app下载 - Actions
- V体育安卓版 - Actions
- "VSports手机版" Actions
- Actions (V体育2025版)
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- "V体育ios版" Actions
- "VSports" Actions
Substances
- Actions (V体育安卓版)
- "VSports最新版本" Actions
- Actions (VSports app下载)
- VSports手机版 - Actions
- "V体育官网入口" Actions
LinkOut - more resources
VSports在线直播 - Full Text Sources
Other Literature Sources
Medical

