Paraoxonase 2 overexpression inhibits tumor development in a mouse model of ovarian cancer
- PMID: 29531225
- PMCID: PMC5847560
- DOI: 10.1038/s41419-018-0395-2
V体育ios版 - Paraoxonase 2 overexpression inhibits tumor development in a mouse model of ovarian cancer
V体育安卓版 - Abstract
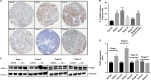
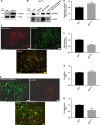
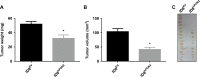
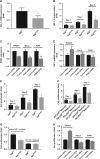
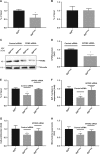
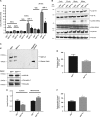
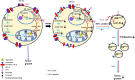
Ovarian cancer (OC) is most lethal malignancy among all gynecological cancer. Large bodies of evidences suggest that mitochondrial-derived ROS play a critical role in the development and progression of OC. Paraoxonase 2 (PON2) is a membrane-associated lactonase with anti-oxidant properties. PON2 deficiency aggravates mitochondrial ROS formation, systemic inflammation, and atherosclerosis. The role of PON2 in cancer development remains unknown. In this report, in human, we identified that PON2 expression is higher in early stages (but not in late stages) of OC when compared to normal tissue. Using a mouse xenograft model of OC, we demonstrate that overexpression of PON2 prevents tumor formation VSports手机版. Mechanistically, PON2 decreases OC cell proliferation by inhibiting insulin like growth factor-1 (IGF-1) expression and signaling. Intriguingly, PON2 reduces c-Jun-mediated transcriptional activation of IGF-1 gene by decreasing mitochondrial superoxide generation. In addition, PON2 impairs insulin like growth factor-1 receptor (IGF-1R) signaling in OC cells by altering cholesterol homeostasis, which resulted in reduced caveolin-1/IGF-1R interaction and IGF-1R phosphorylation. Taken together, we report for the first time that PON2 acts as a tumor suppressor in the early stage of OC by reducing IGF-1 production and its signaling, indicating PON2 activation might be a fruitful strategy to inhibit early stage ovarian tumor. .
Conflict of interest statement
Conflict of interest statement: A. M. F. , and S. T. R. are principals in Bruin Pharma. A. M. F V体育安卓版. is an officer in Bruin Pharma.
Figures
VSports最新版本 - References
-
- Yang Y, et al. Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J. Cell. Physiol. 2016;231:2570–2581. doi: 10.1002/jcp.25349. - DOI (VSports在线直播) - PubMed
-
- Hu Y, et al. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. - V体育官网入口 - DOI - PubMed
"V体育ios版" Publication types
- Actions (VSports注册入口)
MeSH terms (VSports注册入口)
- V体育安卓版 - Actions
- V体育平台登录 - Actions
- VSports app下载 - Actions
- Actions (V体育官网)
- "V体育ios版" Actions
- VSports - Actions
- Actions (VSports)
- "V体育平台登录" Actions
- "V体育ios版" Actions
- V体育安卓版 - Actions
- "V体育2025版" Actions
- "V体育2025版" Actions
Substances
- V体育安卓版 - Actions
- "V体育安卓版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

