Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors
- PMID: 29467321
- PMCID: "V体育平台登录" PMC5916243
- DOI: 10.1172/jci.insight.92352
Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors
Abstract
Background: Constitutive activation of ERK1/2 occurs in various cancers, and its reactivation is a well-described resistance mechanism to MAPK inhibitors. ERK inhibitors may overcome the limitations of MAPK inhibitor blockade. The dual mechanism inhibitor SCH772984 has shown promising preclinical activity across various BRAFV600/RAS-mutant cancer cell lines and human cancer xenografts. VSports手机版.
Methods: We have developed an orally bioavailable ERK inhibitor, MK-8353; conducted preclinical studies to demonstrate activity, pharmacodynamic endpoints, dosing, and schedule; completed a study in healthy volunteers (P07652); and subsequently performed a phase I clinical trial in patients with advanced solid tumors (MK-8353-001). In the P07652 study, MK-8353 was administered as a single dose in 10- to 400-mg dose cohorts, whereas in the MK-8353-001 study, MK-8353 was administered in 100- to 800-mg dose cohorts orally twice daily. Safety, tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity were analyzed. V体育安卓版.
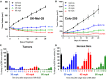
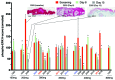
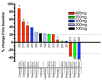
Results: MK-8353 exhibited comparable potency with SCH772984 across various preclinical cancer models. Forty-eight patients were enrolled in the P07652 study, and twenty-six patients were enrolled in the MK-8353-001 study. Adverse events included diarrhea (44%), fatigue (40%), nausea (32%), and rash (28%). Dose-limiting toxicity was observed in the 400-mg and 800-mg dose cohorts V体育ios版. Sufficient exposure to MK-8353 was noted that correlated with biological activity in preclinical data. Three of fifteen patients evaluable for treatment response in the MK-8353-001 study had partial response, all with BRAFV600-mutant melanomas. .
Conclusion: MK-8353 was well tolerated up to 400 mg twice daily and exhibited antitumor activity in patients with BRAFV600-mutant melanoma. However, antitumor activity was not particularly correlated with pharmacodynamic parameters. VSports最新版本.
Trial registration: ClinicalTrials V体育平台登录. gov NCT01358331. .
Funding: Merck Sharp & Dohme Corp. , a subsidiary of Merck & Co. Inc. , and NIH (P01 CA168585 and R35 CA197633) VSports注册入口. .
Keywords: Cancer; Clinical Trials; Melanoma; Oncology; Signal transduction. V体育官网入口.
Figures



VSports注册入口 - References
-
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–3121. doi: 10.1038/sj.onc.1210394. - DOI (VSports注册入口) - PubMed
-
- Ahronian LG, et al. Clinical acquired resistance to raf inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov. 2015;5(4):358–367. doi: 10.1158/2159-8290.CD-14-1518. - DOI - PMC - PubMed
Publication types
- VSports手机版 - Actions
MeSH terms
- Actions (V体育平台登录)
- "V体育官网入口" Actions
- "V体育官网" Actions
- V体育官网入口 - Actions
- VSports app下载 - Actions
- "VSports手机版" Actions
- Actions (V体育安卓版)
- Actions (VSports手机版)
- Actions (V体育ios版)
- Actions (VSports在线直播)
- "V体育2025版" Actions
- V体育官网 - Actions
- "V体育官网入口" Actions
- "VSports app下载" Actions
- "V体育官网" Actions
- Actions (V体育ios版)
- Actions (V体育平台登录)
- VSports手机版 - Actions
- Actions (V体育ios版)
- VSports在线直播 - Actions
- "V体育ios版" Actions
Substances
- "V体育平台登录" Actions
- Actions (VSports最新版本)
- Actions (V体育官网)
- "VSports最新版本" Actions
- V体育官网入口 - Actions
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
VSports在线直播 - Other Literature Sources
V体育平台登录 - Medical
Molecular Biology Databases (V体育2025版)
Miscellaneous

