A Small-Molecule Inhibitor of Human DNA Polymerase η Potentiates the Effects of Cisplatin in Tumor Cells
- PMID: 29345908
- PMCID: PMC7312786
- DOI: 10.1021/acs.biochem.7b01176
A Small-Molecule Inhibitor of Human DNA Polymerase η Potentiates the Effects of Cisplatin in Tumor Cells
Abstract
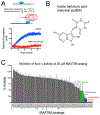
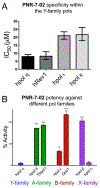
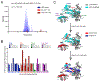
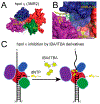
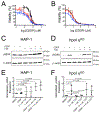
Translesion DNA synthesis (TLS) performed by human DNA polymerase eta (hpol η) allows tolerance of damage from cis-diamminedichloroplatinum(II) (CDDP or cisplatin). We have developed hpol η inhibitors derived from N-aryl-substituted indole barbituric acid (IBA), indole thiobarbituric acid (ITBA), and indole quinuclidine scaffolds and identified 5-((5-chloro-1-(naphthalen-2-ylmethyl)-1H-indol-3-yl)methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (PNR-7-02), an ITBA derivative that inhibited hpol η activity with an IC50 value of 8 μM and exhibited 5-10-fold specificity for hpol η over replicative pols. We conclude from kinetic analyses, chemical footprinting assays, and molecular docking that PNR-7-02 binds to a site on the little finger domain and interferes with the proper orientation of template DNA to inhibit hpol η. A synergistic increase in CDDP toxicity was observed in hpol η-proficient cells co-treated with PNR-7-02 (combination index values = 0 VSports手机版. 4-0. 6). Increased γH2AX formation accompanied treatment of hpol η-proficient cells with CDDP and PNR-7-02. Importantly, PNR-7-02 did not impact the effect of CDDP on cell viability or γH2AX in hpol η-deficient cells. In summary, we observed hpol η-dependent effects on DNA damage/replication stress and sensitivity to CDDP in cells treated with PNR-7-02. The ability to employ a small-molecule inhibitor of hpol η to improve the cytotoxic effect of CDDP may aid in the development of more effective chemotherapeutic strategies. .
"VSports手机版" Conflict of interest statement
The authors declare no competing financial interest.
"V体育平台登录" Figures
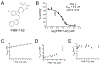
References
-
- Friedberg EC, Aguilera A, Gellert M, Hanawalt PC, Hays JB, Lehmann AR, Lindahl T, Lowndes N, Sarasin A, and Wood RD (2006) DNA repair: from molecular mechanism to human disease. DNA Repair 5, 986–996. - PubMed
-
- Chang DJ, and Cimprich KA (2009) DNA damage tolerance: when it’s OK to make mistakes. Nat. Chem. Biol 5, 82–90. - PMC (V体育官网入口) - PubMed
-
- Xie K, Doles J, Hemann MT, and Walker GC (2010) Error-prone translesion synthesis mediates acquired chemoresistance. Proc. Natl. Acad. Sci U. S. A 107, 20792–20797. - V体育安卓版 - PMC - PubMed
-
- Albertella MR, Green CM, Lehmann AR, and O’Connor MJ (2005) A role for polymerase eta in the cellular tolerance to cisplatin-induced damage. Cancer Res. 65, 9799–9806. - PubMed
-
- Albertella MR, Lau A, and O’Connor MJ (2005) The overexpression of specialized DNA polymerases in cancer. DNA Repair 4, 583–593. - PubMed
Publication types
MeSH terms
- Actions (VSports手机版)
- Actions (V体育安卓版)
- "VSports手机版" Actions
- "V体育2025版" Actions
- "V体育ios版" Actions
- Actions (VSports最新版本)
Substances
- V体育安卓版 - Actions
- VSports在线直播 - Actions
- "VSports app下载" Actions
- V体育官网 - Actions
- Actions (V体育官网入口)
Grants and funding
LinkOut - more resources (VSports手机版)
Full Text Sources
V体育2025版 - Other Literature Sources

