Essential roles of aspartate aminotransferase 1 and vesicular glutamate transporters in β-cell glutamate signaling for incretin-induced insulin secretion
- PMID: 29091932
- PMCID: "VSports" PMC5665537
- DOI: 10.1371/journal.pone.0187213
VSports - Essential roles of aspartate aminotransferase 1 and vesicular glutamate transporters in β-cell glutamate signaling for incretin-induced insulin secretion
VSports最新版本 - Abstract
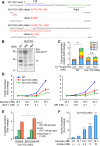
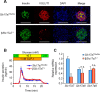
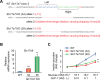
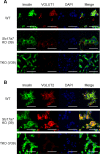
Incretins (GLP-1 and GIP) potentiate insulin secretion through cAMP signaling in pancreatic β-cells in a glucose-dependent manner. We recently proposed a mechanistic model of incretin-induced insulin secretion (IIIS) that requires two critical processes: 1) generation of cytosolic glutamate through the malate-aspartate (MA) shuttle in glucose metabolism and 2) glutamate transport into insulin granules by cAMP signaling to promote insulin granule exocytosis. To directly prove the model, we have established and characterized CRISPR/Cas9-engineered clonal mouse β-cell lines deficient for the genes critical in these two processes: aspartate aminotransferase 1 (AST1, gene symbol Got1), a key enzyme in the MA shuttle, which generates cytosolic glutamate, and the vesicular glutamate transporters (VGLUT1, VGLUT2, and VGLUT3, gene symbol Slc17a7, Slc17a6, and Slc17a8, respectively), which participate in glutamate transport into secretory vesicles VSports手机版. Got1 knockout (KO) β-cell lines were defective in cytosolic glutamate production from glucose and showed impaired IIIS. Unexpectedly, different from the previous finding that global Slc17a7 KO mice exhibited impaired IIIS from pancreatic islets, β-cell specific Slc17a7 KO mice showed no significant impairment in IIIS, as assessed by pancreas perfusion experiment. Single Slc17a7 KO β-cell lines also retained IIIS, probably due to compensatory upregulation of Slc17a6. Interestingly, triple KO of Slc17a7, Slc17a6, and Slc17a8 diminished IIIS, which was rescued by exogenously introduced wild-type Slc17a7 or Slc17a6 genes. The present study provides direct evidence for the essential roles of AST1 and VGLUTs in β-cell glutamate signaling for IIIS and also shows the usefulness of the CRISPR/Cas9 system for studying β-cells by simultaneous disruption of multiple genes. .
Conflict of interest statement
VSports手机版 - Figures
References
-
- Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52: 739–751. doi: 10.1007/s00125-009-1314-y - DOI - PubMed
-
- Yokoi N, Gheni G, Takahashi H, Seino S. β-cell glutamate signaling: Its role in incretin-induced insulin secretion. J Diabetes Investig. 2016;7: 38–43. doi: 10.1111/jdi.12468 - DOI - PMC - PubMed
-
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3: 153–165. doi: 10.1016/j.cmet.2006.01.004 - DOI - PubMed
-
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87: 1409–1439. doi: VSports注册入口 - 10.1152/physrev.00034.2006 - DOI - PubMed
-
- Siegel EG, Creutzfeldt W. Stimulation of insulin release in isolated rat islets by GIP in physiological concentrations and its relation to islet cyclic AMP content. Diabetologia. 1985;28: 857–861. - PubMed
MeSH terms (VSports在线直播)
- Actions (VSports在线直播)
- VSports注册入口 - Actions
- "V体育2025版" Actions
- Actions (VSports注册入口)
- VSports手机版 - Actions
- Actions (V体育官网)
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- "VSports" Actions
Substances
- "V体育平台登录" Actions
- VSports在线直播 - Actions
"VSports手机版" LinkOut - more resources
Full Text Sources (V体育ios版)
Other Literature Sources
Medical
"V体育官网" Molecular Biology Databases
Research Materials

