Coding and noncoding landscape of extracellular RNA released by human glioma stem cells
- PMID: 29074968
- PMCID: PMC5658400
- DOI: VSports - 10.1038/s41467-017-01196-x
Coding and noncoding landscape of extracellular RNA released by human glioma stem cells
"VSports app下载" Abstract
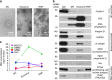
Tumor-released RNA may mediate intercellular communication and serve as biomarkers. Here we develop a protocol enabling quantitative, minimally biased analysis of extracellular RNAs (exRNAs) associated with microvesicles, exosomes (collectively called EVs), and ribonucleoproteins (RNPs). The exRNA complexes isolated from patient-derived glioma stem-like cultures exhibit distinct compositions, with microvesicles most closely reflecting cellular transcriptome. exRNA is enriched in small ncRNAs, such as miRNAs in exosomes, and precisely processed tRNA and Y RNA fragments in EVs and exRNPs. EV-enclosed mRNAs are mostly fragmented, and UTRs enriched; nevertheless, some full-length mRNAs are present. Overall, there is less than one copy of non-rRNA per EV VSports手机版. Our results suggest that massive EV/exRNA uptake would be required to ensure functional impact of transferred RNA on brain recipient cells and predict the most impactful miRNAs in such conditions. This study also provides a catalog of diverse exRNAs useful for biomarker discovery and validates its feasibility on cerebrospinal fluid. .
"VSports注册入口" Conflict of interest statement
X. O V体育安卓版. B. is a consulting member of the Scientific Advisory Board of Evox Therapeutics, Ltd. and Exocyte Therapeutics, Ltd. The remaining authors declare no competing financial interests.
Figures







References
VSports手机版 - Publication types
- Actions (VSports app下载)
MeSH terms
- Actions (VSports手机版)
- "V体育安卓版" Actions
- V体育ios版 - Actions
- V体育ios版 - Actions
- VSports - Actions
- VSports注册入口 - Actions
- VSports注册入口 - Actions
Substances
- VSports注册入口 - Actions
- "V体育官网" Actions
Grants and funding
LinkOut - more resources (VSports app下载)
Full Text Sources
Other Literature Sources
Molecular Biology Databases

