"VSports" Dual-Responsive Molecular Probe for Tumor Targeted Imaging and Photodynamic Therapy
- PMID: 28638467
- PMCID: "V体育ios版" PMC5479268
- DOI: "VSports" 10.7150/thno.18437
Dual-Responsive Molecular Probe for Tumor Targeted Imaging and Photodynamic Therapy
Abstract
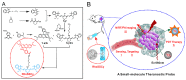
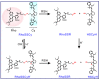
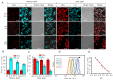
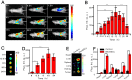
The precision oncology significantly relies on the development of multifunctional agents to integrate tumor targeting, imaging and therapeutics VSports手机版. In this study, a first small-molecule theranostic probe, RhoSSCy is constructed by conjugating 5'-carboxyrhodamines (Rho) and heptamethine cyanine IR765 (Cy) using a reducible disulfide linker and pH tunable amino-group to realize thiols/pH dual sensing. In vitro experiments verify that RhoSSCy is highly sensitive for quantitative analysis and imaging intracellular pH gradient and biothiols. Furthermore, RhoSSCy shows superb tumor targeted dual-modal imaging via near-infrared fluorescence (NIRF) and photoacoustic (PA). Importantly, RhoSSCy also induces strongly reactive oxygen species for tumor photodynamic therapy (PDT) with robust antitumor activity both in vitro and in vivo. Such versatile small-molecule theranostic probe may be promising for tumor targeted imaging and precision therapy. .
Keywords: Dual-stimuli sensing; NIRF/PA imaging; Photodynamic therapy. ; Small-molecule theranostic probe; tumor targeting. V体育安卓版.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Bardhan R, Lal S, Joshi A. et al. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res. 2011;44(1):936–946. - VSports在线直播 - PMC - PubMed
-
- Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine. 2008;3(2):137–140. - PubMed
-
- Sharma R, Mody N, Agrawal U. et al. Theranostic nanomedicine; a next generation platform for cancer diagnosis and therapy. Mini Rev Med Chem. 2016;16(999):1. - "VSports最新版本" PubMed
Publication types
"VSports app下载" MeSH terms
- "VSports" Actions
- VSports最新版本 - Actions
- Actions (VSports注册入口)
- "VSports在线直播" Actions
- VSports app下载 - Actions
- VSports - Actions
- Actions (VSports手机版)
- "VSports最新版本" Actions
- "VSports" Actions
"V体育ios版" Substances
LinkOut - more resources
V体育官网入口 - Full Text Sources
Other Literature Sources

