Temporary, Systemic Inhibition of the WNT/β-Catenin Pathway promotes Regenerative Cardiac Repair following Myocardial Infarct (VSports注册入口)
- PMID: 28042617
- PMCID: PMC5193163
- DOI: "VSports在线直播" 10.16966/2472-6990.111
Temporary, Systemic Inhibition of the WNT/β-Catenin Pathway promotes Regenerative Cardiac Repair following Myocardial Infarct
Abstract
Aims: The WNT/β-catenin pathway is temporarily activated in the heart following myocardial infarction (MI). Despite data from genetic models indicating both positive and negative roles for the WNT pathway depending on the model used, the effect of therapeutic inhibition of WNT pathway on post-injury outcome and the cellular mediators involved are not completely understood VSports手机版. Using a newly available, small molecule, GNF-6231, which averts WNT pathway activation by blocking secretion of all WNT ligands, we sought to investigate whether therapeutic inhibition of the WNT pathway temporarily after infarct can mitigate post injury cardiac dysfunction and fibrosis and the cellular mechanisms responsible for the effects. .
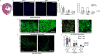
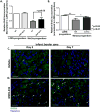
Methods and results: Pharmacologic inhibition of the WNT pathway by post-MI intravenous injection of GNF-6231 in C57Bl/6 mice significantly reduced the decline in cardiac function (Fractional Shortening at day 30: 38. 71 ± 4. 13% in GNF-6231 treated vs. 34. 89 ± 4. 86% in vehicle-treated), prevented adverse cardiac remodeling, and reduced infarct size (9. 07 ± 3 V体育安卓版. 98% vs. 17. 18 ± 4. 97%). WNT inhibition augmented proliferation of interstitial cells, particularly in the distal myocardium, inhibited apoptosis of cardiomyocytes, and reduced myofibroblast proliferation in the peri-infarct region. In vitro studies showed that WNT inhibition increased proliferation of Sca1+ cardiac progenitors, improved survival of cardiomyocytes, and inhibited collagen I synthesis by cardiac myofibroblasts. .
Conclusion: Systemic, temporary pharmacologic inhibition of the WNT pathway using an orally bioavailable drug immediately following MI resulted in improved function, reduced adverse remodeling and reduced infarct size in mice. Therapeutic WNT inhibition affected multiple aspects of infarct repair: it promoted proliferation of cardiac progenitors and other interstitial cells, inhibited myofibroblast proliferation, improved cardiomyocyte survival, and reduced collagen I gene expression by myofibroblasts. Our data point to a promising role for WNT inhibitory therapeutics as a new class of drugs to drive post-MI repair and prevent heart failure V体育ios版. .
Keywords: Myocardial infarct; Regenerative cardiac repair; WNT/β-Catenin Pathway VSports最新版本. .
Conflict of interest statement
Conflicts of Interests JL and JLH are employees of the Novartis Research Foundation. PPY is listed as inventor for a WNT inhibitory topical therapeutic V体育平台登录.
Figures (VSports app下载)
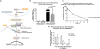




References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. - PubMed
-
- Mill JG, Stefanon I, dos Santos L, Baldo MP. Remodeling in the ischemic heart: the stepwise progression for heart failure. Braz J Med Biol Res. 2011;44:890–898. - PubMed (V体育ios版)
-
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circ. 2000;101:2981–2988. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
