In vivo anti-tumor activity of the PARP inhibitor niraparib in homologous recombination deficient and proficient ovarian carcinoma
- PMID: 27614696
- PMCID: "V体育ios版" PMC5370566
- DOI: 10.1016/j.ygyno.2016.08.328
"V体育ios版" In vivo anti-tumor activity of the PARP inhibitor niraparib in homologous recombination deficient and proficient ovarian carcinoma
Abstract (VSports手机版)
Objective: Poly(ADP-ribose) polymerase (PARP) inhibitors have yielded encouraging responses in high-grade serous ovarian carcinomas (HGSOCs), but the optimal treatment setting remains unknown. We assessed the effect of niraparib on HGSOC patient-derived xenograft (PDX) models as well as the relationship between certain markers of homologous recombination (HR) status, including BRCA1/2 mutations and formation of RAD51 foci after DNA damage, and response of these PDXs to niraparib in vivo. VSports手机版.
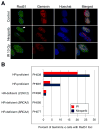
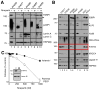
Methods: Massively parallel sequencing was performed on HGSOCs to identify mutations contributing to HR deficiency. HR pathway integrity was assessed using fluorescence microscopy-based RAD51 focus formation assays. Effects of niraparib (MK-4827) on treatment-naïve PDX tumor growth as monotherapy, in combination with carboplatin/paclitaxel, and as maintenance therapy were assessed by transabdominal ultrasound. Niraparib responses were correlated with changes in levels of poly(ADP-ribose), PARP1, and repair proteins by western blotting. V体育安卓版.
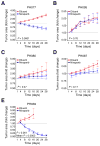
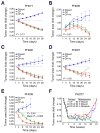
Results: Five PDX models were evaluated in vivo. Tumor regressions were induced by single-agent niraparib in one of two PDX models with deleterious BRCA2 mutations and in a PDX with RAD51C promoter methylation. Diminished formation of RAD51 foci failed to predict response, but Artemis loss was associated with resistance V体育ios版. Niraparib generally failed to enhance responses to carboplatin/paclitaxel chemotherapy, but maintenance niraparib therapy delayed progression in a BRCA2-deficient PDX. .
Conclusions: Mutations in HR genes are neither necessary nor sufficient to predict response to niraparib. Assessment of repair status through multiple complementary assays is needed to guide PARP inhibitor therapy, design future clinical trials and identify ovarian cancer patients most likely to benefit from PARP inhibition. VSports最新版本.
Keywords: BRCA; DNA repair; Homologous recombination; Niraparib; Ovarian cancer; PARP inhibitors; Xenografts. V体育平台登录.
Copyright © 2016 Elsevier Inc. All rights reserved. VSports注册入口.
Conflict of interest statement
Authors' disclosure of potential conflicts of interest: PH was an unpaid consultant for Merck and received research funding from Merck and Tesaro.
Dr. Becker reports personal fees from Ovarian Cancer Tumorgrafts - Avatar System, outside the submitted work; Dr. Haluska reports non-financial support from Tesaro, during the conduct of the study through the supply of niraparib; and intellectual property (non-patent) involving the use of the PDX models employed in this work, which includes the awarding of royalties. Dr. Kaufmann reports grants from National Cancer Institute and Ovarian Cancer Research Fund during the conduct of the study. In addition, Mayo Clinic has a patent related to methods for assessing responsiveness to PARP inhibitors on which Dr VSports在线直播. Kaufmann is a co-inventor. Dr. Weroha reports grants from Tesaro, during the conduct of the study.
"V体育ios版" Figures
References
-
- Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–1782. - "VSports" PMC - PubMed
-
- Wickramanyake A, Bernier G, Pennil C, Casadei S, Agnew KJ, Stray SM, et al. Loss of function germline mutations in RAD51D in women with ovarian carcinoma. Gynecol Oncol. 2012;127:552–555. - "VSports" PMC - PubMed
MeSH terms
- "V体育ios版" Actions
- V体育ios版 - Actions
- "VSports最新版本" Actions
- V体育2025版 - Actions
- "VSports" Actions
Substances
- "VSports" Actions
- Actions (VSports)
- Actions (V体育官网入口)
- Actions (VSports最新版本)
Grants and funding (VSports注册入口)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous (V体育官网入口)

