Study protocol for a phase III multicentre, randomised, open-label, blinded-end point trial to evaluate the efficacy and safety of immunoglobulin plus cyclosporin A in patients with severe Kawasaki disease (KAICA Trial)
- PMID: 26628527
- PMCID: V体育官网 - PMC4679944
- DOI: V体育安卓版 - 10.1136/bmjopen-2015-009562
Study protocol for a phase III multicentre, randomised, open-label, blinded-end point trial to evaluate the efficacy and safety of immunoglobulin plus cyclosporin A in patients with severe Kawasaki disease (KAICA Trial)
Abstract (VSports在线直播)
Introduction: Kawasaki disease (KD) is an acute, self-limited vasculitis of unknown aetiology that predominantly affects infants and young children. We hypothesise that cyclosporin A (CsA) may be effective in treating KD by regulating the Ca(2+)/NFAT signalling pathway. This trial compares the current standard therapy of intravenous immunoglobulin (IVIG) and the combined IVIG+CsA therapy in paediatric patients with severe KD. VSports手机版.
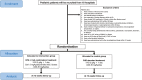
Methods and analysis: This trial is a phase III, multicentre, randomised, open-label, blinded-end point trial that evaluates the efficacy and safety of IVIG+CsA therapy V体育安卓版. Patients with severe KD who satisfy the eligibility criteria are randomised (1:1) to receive either CsA (5 mg/kg/day for 5 days; Neoral) plus high-dose IVIG (2 g/kg for 24 h and aspirin 30 mg/kg/day), or high-dose IVIG alone (2 g/kg for 24 h and aspirin 30 mg/kg/day). The primary end point is the frequency of occurrence of coronary artery abnormalities during the trial period. An independent end point review committee will be in charge of the trial assessment. .
Ethics and dissemination: The protocol was approved by the Institutional Review Board of each institution. The trial was notified and registered at the Pharmaceutical and Medical Devices Agency, in Japan V体育ios版. The trial is currently on-going and is scheduled to finish in April 2017. The findings will be disseminated through peer-reviewed publications and conference presentations. .
Trial registration number: JMA-IIA00174; Pre-results VSports最新版本. .
Keywords: CLINICAL PHARMACOLOGY. V体育平台登录.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www VSports注册入口. bmj. com/company/products-services/rights-and-licensing/ .
Figures
References
-
- Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967;16:178. - PubMed
-
- Nakamura Y, Yashiro M, Uehara R et al. . Epidemiologic features of Kawasaki disease in Japan: results of the 2007–2008 nationwide survey. J Epidemiol 2010;20:302 10.2188/jea.JE20090180 - "VSports注册入口" DOI - PMC - PubMed
-
- Newburger JW, Takahashi M, Burns JC et al. . The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med 1986;315:341–7. 10.1056/NEJM198608073150601 - VSports在线直播 - DOI - PubMed
Publication types
- "V体育官网入口" Actions
MeSH terms
- "V体育ios版" Actions
- "V体育官网" Actions
- VSports手机版 - Actions
- "VSports在线直播" Actions
- VSports手机版 - Actions
- "V体育安卓版" Actions
- VSports - Actions
"VSports注册入口" Substances
- Actions (V体育官网)
- "VSports注册入口" Actions
- VSports注册入口 - Actions
LinkOut - more resources
"VSports最新版本" Full Text Sources
Other Literature Sources
Medical
Miscellaneous