Inhibition of Osteoclastogenesis and Bone Resorption in vitro and in vivo by a prenylflavonoid xanthohumol from hops
- PMID: 26620037
- PMCID: PMC4664947
- DOI: 10.1038/srep17605
V体育官网入口 - Inhibition of Osteoclastogenesis and Bone Resorption in vitro and in vivo by a prenylflavonoid xanthohumol from hops
Abstract
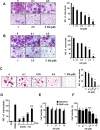
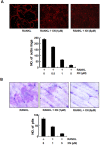
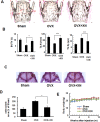
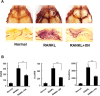
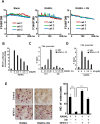
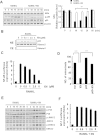
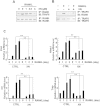
Excessive RANKL signaling leads to superfluous osteoclast formation and bone resorption, is widespread in the pathologic bone loss and destruction. Therefore, targeting RANKL or its signaling pathway has been a promising and successful strategy for this osteoclast-related diseases. In this study, we examined the effects of xanthohumol (XN), an abundant prenylflavonoid from hops plant, on osteoclastogenesis, osteoclast resorption, and RANKL-induced signaling pathway using both in vitro and in vivo assay systems. In mouse and human, XN inhibited osteoclast differentiation and osteoclast formation at the early stage. Furthermore, XN inhibited osteoclast actin-ring formation and bone resorption in a dose-dependent manner. In ovariectomized-induced bone loss mouse model and RANKL-injection-induced bone resorption model, we found that administration of XN markedly inhibited bone loss and resorption by suppressing osteoclast activity. At the molecular level, XN disrupted the association of RANK and TRAF6, resulted in the inhibition of NF-κB and Ca(2+)/NFATc1 signaling pathway during osteoclastogenesis. As a results, XN suppressed the expression of osteoclastogenesis-related marker genes, including CtsK, Nfatc1, Trap, Ctr VSports手机版. Therefore, our data demonstrated that XN inhibits osteoclastogenesis and bone resorption through RANK/TRAF6 signaling pathways. XN could be a promising drug candidate in the treatment of osteoclast-related diseases such as postmenopausal osteoporosis. .
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Lee J. H. et al. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol Pharmacol 77, 17–25 (2010). - PubMed
-
- Vives V. et al. Pharmacological inhibition of Dock5 prevents osteolysis by affecting osteoclast podosome organization while preserving bone formation. Nat Commun 6, 6218, 10.1038/ncomms7218 (2015). - "V体育2025版" DOI - PubMed
-
- Cummings S. R. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361, 756–765 (2009). - "VSports最新版本" PubMed
"VSports在线直播" Publication types
"VSports注册入口" MeSH terms
- Actions (VSports app下载)
- VSports - Actions
- "V体育官网" Actions
- Actions (V体育平台登录)
- "V体育ios版" Actions
- V体育2025版 - Actions
- V体育官网 - Actions
- VSports在线直播 - Actions
- Actions (VSports app下载)
Substances
- Actions (VSports app下载)
- "V体育官网入口" Actions
- VSports注册入口 - Actions
- VSports - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育ios版" Research Materials
Miscellaneous

