Comprehensive investigation of oncogenic driver mutations in Chinese non-small cell lung cancer patients
- PMID: 26486077
- PMCID: "VSports手机版" PMC4741453
- DOI: 10.18632/oncotarget.5549
Comprehensive investigation of oncogenic driver mutations in Chinese non-small cell lung cancer patients
Abstract
Purpose: To determine the frequency of driver mutations in Chinese non-small cell lung cancer (NSCLC) patients. VSports手机版.
Methods: Comprehensive mutational analysis was performed in 1356 lung adenocarcinoma, 503 squamous cell carcinoma, 57 adenosquamous lung carcinoma, 19 large cell carcinoma and 8 sarcomatoid carcinoma. The effect of EGFR tyrosine kinase inhibitors (TKIs) on EGFR-mutated lung adenocarcinoma patients after disease recurrence was investigated. V体育安卓版.
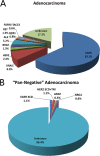
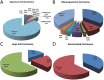
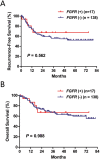
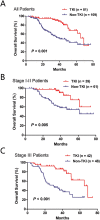
Results: Mutations in EGFR kinase domain, HER2 kinase domain, KRAS, BRAF, ALK, ROS1 and RET were mutually exclusive. In lung adenocarcinoma cases "pan-negative" for the seven above-mentioned driver mutations, we also detected two oncogenic EGFR extracellular domain mutations (A289D and R324L), two HER2 extracellular and transmembrane domain mutations (S310Y and V659E), one ARAF S214C mutation and two CD74-NRG1 fusions. Six (1. 2%) FGFR3 activating mutations were identified in lung squamous cell carcinoma (five S249C and one R248C). There were three (15. 8%) EGFR mutations and four (21. 1%) KRAS mutations in large cell carcinoma. Three (37. 5%) KRAS mutations were detected in sarcomatoid carcinoma. In EGFR-mutated lung adenocarcinoma patients who experienced disease recurrence, treatment with EGFR TKIs was an independent predictor of better overall survival (HR = 0. 299, 95% CI: 0. 172-0. 519, P < 0. 001) V体育ios版. .
Conclusion: We determined the frequency of driver mutations in a large series of Chinese NSCLC patients. EGFR TKIs might improve the survival outcomes of EGFR-mutated lung adenocarcinoma patients who experienced disease recurrence VSports最新版本. .
Keywords: ERBB; FGFR; driver mutations; non-small cell lung cancer V体育平台登录. .
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures
References
-
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
-
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. - PubMed
Publication types
- Actions (V体育平台登录)
MeSH terms
- Actions (VSports最新版本)
- Actions (VSports注册入口)
- VSports最新版本 - Actions
- V体育安卓版 - Actions
- "VSports app下载" Actions
- Actions (V体育2025版)
- Actions (V体育安卓版)
- VSports - Actions
- "V体育官网" Actions
- Actions (V体育官网)
- VSports手机版 - Actions
- "V体育安卓版" Actions
- VSports在线直播 - Actions
"V体育官网入口" Substances
- "VSports在线直播" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

