Ras-GTP dimers activate the Mitogen-Activated Protein Kinase (MAPK) pathway (VSports注册入口)
- PMID: 26080442
- PMCID: "VSports手机版" PMC4491781
- DOI: "VSports手机版" 10.1073/pnas.1509123112
Ras-GTP dimers activate the Mitogen-Activated Protein Kinase (MAPK) pathway
V体育2025版 - Abstract
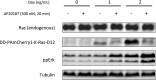
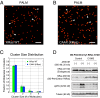
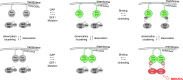
Rat sarcoma (Ras) GTPases regulate cell proliferation and survival through effector pathways including Raf-MAPK, and are the most frequently mutated genes in human cancer. Although it is well established that Ras activity requires binding to both GTP and the membrane, details of how Ras operates on the cell membrane to activate its effectors remain elusive. Efforts to target mutant Ras in human cancers to therapeutic benefit have also been largely unsuccessful VSports手机版. Here we show that Ras-GTP forms dimers to activate MAPK. We used quantitative photoactivated localization microscopy (PALM) to analyze the nanoscale spatial organization of PAmCherry1-tagged KRas 4B (hereafter referred to KRas) on the cell membrane under various signaling conditions. We found that at endogenous expression levels KRas forms dimers, and KRas(G12D), a mutant that constitutively binds GTP, activates MAPK. Overexpression of KRas leads to formation of higher order Ras nanoclusters. Conversely, at lower expression levels, KRas(G12D) is monomeric and activates MAPK only when artificially dimerized. Moreover, dimerization and signaling of KRas are both dependent on an intact CAAX (C, cysteine; A, aliphatic; X, any amino acid) motif that is also known to mediate membrane localization. These results reveal a new, dimerization-dependent signaling mechanism of Ras, and suggest Ras dimers as a potential therapeutic target in mutant Ras-driven tumors. .
Keywords: MAPK signaling; Ras dimer; cancer; single molecule imaging; superresolution microscopy. V体育安卓版.
"V体育2025版" Conflict of interest statement
The authors declare no conflict of interest.
Figures (VSports注册入口)








Comment in
-
Seeing is believing: Ras dimers observed in live cells.Proc Natl Acad Sci U S A. 2015 Aug 11;112(32):9793-4. doi: 10.1073/pnas.1511805112. Epub 2015 Jul 30. Proc Natl Acad Sci U S A. 2015. PMID: 26229079 Free PMC article. No abstract available.
References (V体育平台登录)
-
- Malumbres M, Barbacid M. RAS oncogenes: The first 30 years. Nat Rev Cancer. 2003;3(6):459–465. - PubMed
-
- Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer. 2011;11(11):761–774. - PMC (V体育官网) - PubMed
-
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7(4):295–308. - V体育ios版 - PubMed
Publication types
- VSports app下载 - Actions
- "VSports app下载" Actions
MeSH terms
- "V体育2025版" Actions
- Actions (V体育平台登录)
- Actions (VSports在线直播)
- Actions (V体育平台登录)
- VSports手机版 - Actions
- "V体育ios版" Actions
- VSports - Actions
Substances
- VSports app下载 - Actions
- Actions (V体育平台登录)
- "VSports app下载" Actions
V体育2025版 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育安卓版 - Research Materials
Miscellaneous

