Next-generation clinical trials: Novel strategies to address the challenge of tumor molecular heterogeneity (V体育2025版)
- PMID: 25557400
- PMCID: PMC4402102
- DOI: 10.1016/j.molonc.2014.09.011
Next-generation clinical trials: Novel strategies to address the challenge of tumor molecular heterogeneity
"VSports注册入口" Abstract
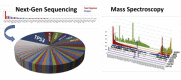
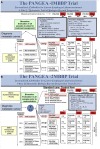
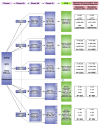
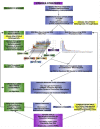
The promise of 'personalized cancer care' with therapies toward specific molecular aberrations has potential to improve outcomes. However, there is recognized heterogeneity within any given tumor-type from patient to patient (inter-patient heterogeneity), and within an individual (intra-patient heterogeneity) as demonstrated by molecular evolution through space (primary tumor to metastasis) and time (after therapy). These issues have become hurdles to advancing cancer treatment outcomes with novel molecularly targeted agents. Classic trial design paradigms are challenged by heterogeneity, as they are unable to test targeted therapeutics against low frequency genomic 'oncogenic driver' aberrations with adequate power. Usual accrual difficulties to clinical trials are exacerbated by low frequencies of any given molecular driver. To address these challenges, there is need for innovative clinical trial designs and strategies implementing novel diagnostic biomarker technologies to account for inter-patient molecular diversity and scarce tissue for analysis. Importantly, there is also need for pre-defined treatment priority algorithms given numerous aberrations commonly observed within any one individual sample VSports手机版. Access to multiple available therapeutic agents simultaneously is crucial. Finally intra-patient heterogeneity through time may be addressed by serial biomarker assessment at the time of tumor progression. This report discusses various 'next-generation' biomarker-driven trial designs and their potentials and limitations to tackle these recognized molecular heterogeneity challenges. Regulatory hurdles, with respect to drug and companion diagnostic development and approval, are considered. Focus is on the 'Expansion Platform Design Types I and II', the latter demonstrated with a first example, 'PANGEA: Personalized Anti-Neoplastics for Gastro-Esophageal Adenocarcinoma'. Applying integral medium-throughput genomic and proteomic assays along with a practical biomarker assessment and treatment algorithm, 'PANGEA' attempts to address the problem of heterogeneity towards successful implementation of molecularly targeted therapies. .
Keywords: Esophagogastric cancer; Esophagus cancer; Expansion Platform Designs; Gastric cancer; Gastroesophageal cancer; Inter-patient heterogeneity; Intra-patient heterogeneity; Molecular heterogeneity; Next-generation clinical trials; PANGEA. V体育安卓版.
Copyright © 2014 The Author. Published by Elsevier B. V. All rights reserved. V体育ios版.
"V体育ios版" Figures




"VSports手机版" References
-
- Abrams, J. , Conley, B. , Mooney, M. , 2014. National cancer institute's precision medicine initiatives for the new national clinical trials network. Am. Soc. Clin. Oncol. Educ. Book. 71–76. - PubMed
-
- Alix-Panabieres, C. , Pantel, K. , 2014. Challenges in circulating tumour cell research. Nat. Rev. Cancer. 14, 623–631. - PubMed
-
- Arena, V. , Pennacchia, I. , Vecchio, F.M. , Carbone, A. , 2013. HER-2 intratumoral heterogeneity. Mod. Pathol. 26, 607–609. - PubMed
-
- Bang, Y.J. , Van Cutsem, E. , Feyereislova, A. , 2010. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 376, 687–697. - PubMed
Publication types
- Actions (VSports最新版本)
MeSH terms
- "VSports app下载" Actions
"V体育2025版" Grants and funding
LinkOut - more resources
VSports在线直播 - Full Text Sources
"VSports注册入口" Other Literature Sources

