Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation (V体育ios版)
- PMID: 25366685
- PMCID: PMC6057749
- DOI: 10.1200/JCO.2014.56.2728
Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation
Abstract
Purpose: Olaparib is an oral poly (ADP-ribose) polymerase inhibitor with activity in germline BRCA1 and BRCA2 (BRCA1/2) -associated breast and ovarian cancers. We evaluated the efficacy and safety of olaparib in a spectrum of BRCA1/2-associated cancers VSports手机版. .
Patients and methods: This multicenter phase II study enrolled individuals with a germline BRCA1/2 mutation and recurrent cancer. Eligibility included ovarian cancer resistant to prior platinum; breast cancer with ≥ three chemotherapy regimens for metastatic disease; pancreatic cancer with prior gemcitabine treatment; or prostate cancer with progression on hormonal and one systemic therapy. Olaparib was administered at 400 mg twice per day. The primary efficacy end point was tumor response rate V体育安卓版. .
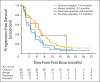
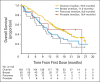
Results: A total of 298 patients received treatment and were evaluable. The tumor response rate was 26. 2% (78 of 298; 95% CI, 21. 3 to 31. 6) overall and 31. 1% (60 of 193; 95% CI, 24. 6 to 38. 1), 12. 9% (eight of 62; 95% CI, 5. 7 to 23. 9), 21. 7% (five of 23; 95% CI, 7. 5 to 43. 7), and 50. 0% (four of eight; 95% CI, 15. 7 to 84. 3) in ovarian, breast, pancreatic, and prostate cancers, respectively. Stable disease ≥ 8 weeks was observed in 42% of patients (95% CI, 36. 0 to 47. 4), including 40% (95% CI, 33. 4 to 47. 7), 47% (95% CI, 34. 0 to 59. 9), 35% (95% CI, 16. 4 to 57. 3), and 25% (95% CI, 3. 2 to 65. 1) of those with ovarian, breast, pancreatic, or prostate cancer, respectively. The most common adverse events (AEs) were fatigue, nausea, and vomiting V体育ios版. Grade ≥ 3 AEs were reported for 54% of patients; anemia was the most common (17%). .
Conclusion: Responses to olaparib were observed across different tumor types associated with germline BRCA1/2 mutations. Olaparib warrants further investigation in confirmatory studies VSports最新版本. .
© 2014 by American Society of Clinical Oncology V体育平台登录. .
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
"VSports注册入口" Comment in
-
The long and winding road.J Clin Oncol. 2015 Jan 20;33(3):229-31. doi: 10.1200/JCO.2014.59.2311. Epub 2014 Dec 1. J Clin Oncol. 2015. PMID: 25452438 No abstract available.
-
To BRCA or Not to PALB.J Clin Oncol. 2015 Aug 10;33(23):2581-2. doi: 10.1200/JCO.2014.60.0585. Epub 2015 Jun 29. J Clin Oncol. 2015. PMID: 26124473 No abstract available.
-
Germline BRCA1/2 Mutations: Are They Good Enough to Determine Who Will Respond to Poly(ADP-Ribose) Polymerase Inhibitor Therapy in Advanced Cancer?J Clin Oncol. 2015 Aug 10;33(23):2582. doi: 10.1200/JCO.2015.61.0576. Epub 2015 Jun 29. J Clin Oncol. 2015. PMID: 26124479 No abstract available.
-
Reply to M.G. McNamara et al and M.S. Copur et al.J Clin Oncol. 2015 Aug 10;33(23):2583-4. doi: 10.1200/JCO.2015.61.8298. Epub 2015 Jun 29. J Clin Oncol. 2015. PMID: 26124483 No abstract available.
-
[POLO study].Bull Cancer. 2019 Sep;106(9):717-718. doi: 10.1016/j.bulcan.2019.07.003. Epub 2019 Aug 13. Bull Cancer. 2019. PMID: 31420091 French. No abstract available.
References
-
- Murai J Huang SY Das BB , etal: Trapping of PARP1 and PARP2 by clinical PARP inhibitors Cancer Res 72: 5588– 5599,2012. - PMC (V体育安卓版) - PubMed
-
- Bryant HE Schultz N Thomas HD , etal: Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase Nature 434: 913– 917,2005. - PubMed
-
- Farmer H McCabe N Lord CJ , etal: Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy Nature 434: 917– 921,2005. - "V体育官网入口" PubMed
-
- Tutt A Robson M Garber JE , etal: Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial Lancet 376: 235– 244,2010. - V体育官网 - PubMed
-
- Audeh MW Carmichael J Penson RT , etal: Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial Lancet 376: 245– 251,2010. - VSports手机版 - PubMed
Publication types (V体育官网入口)
MeSH terms
- Actions (VSports注册入口)
- "V体育ios版" Actions
- Actions (V体育官网)
- Actions (V体育平台登录)
- Actions (V体育官网入口)
- Actions (VSports在线直播)
- Actions (V体育安卓版)
- Actions (VSports手机版)
- "VSports最新版本" Actions
- V体育ios版 - Actions
- "VSports在线直播" Actions
- Actions (V体育安卓版)
- Actions (VSports注册入口)
- "VSports最新版本" Actions
Substances
- "V体育2025版" Actions
- V体育官网 - Actions
- Actions (VSports在线直播)
- Actions (V体育安卓版)
Grants and funding (V体育官网入口)
"VSports" LinkOut - more resources
"V体育官网入口" Full Text Sources
Other Literature Sources
Medical
Miscellaneous