Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype
- PMID: 24492615
- PMCID: "V体育平台登录" PMC3953299
- DOI: 10.1074/jbc.M113.522037
"VSports app下载" Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype
"VSports" Abstract
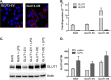
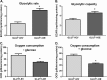
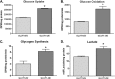
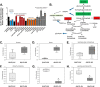
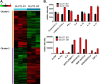
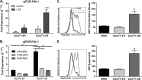
Glucose is a critical component in the proinflammatory response of macrophages (MΦs). However, the contribution of glucose transporters (GLUTs) and the mechanisms regulating subsequent glucose metabolism in the inflammatory response are not well understood. Because MΦs contribute to obesity-induced inflammation, it is important to understand how substrate metabolism may alter inflammatory function. We report that GLUT1 (SLC2A1) is the primary rate-limiting glucose transporter on proinflammatory-polarized MΦs. Furthermore, in high fat diet-fed rodents, MΦs in crown-like structures and inflammatory loci in adipose and liver, respectively, stain positively for GLUT1. We hypothesized that metabolic reprogramming via increased glucose availability could modulate the MΦ inflammatory response. To increase glucose uptake, we stably overexpressed the GLUT1 transporter in RAW264. 7 MΦs (GLUT1-OE MΦs). Cellular bioenergetics analysis, metabolomics, and radiotracer studies demonstrated that GLUT1 overexpression resulted in elevated glucose uptake and metabolism, increased pentose phosphate pathway intermediates, with a complimentary reduction in cellular oxygen consumption rates. Gene expression and proteome profiling analysis revealed that GLUT1-OE MΦs demonstrated a hyperinflammatory state characterized by elevated secretion of inflammatory mediators and that this effect could be blunted by pharmacologic inhibition of glycolysis. Finally, reactive oxygen species production and evidence of oxidative stress were significantly enhanced in GLUT1-OE MΦs; antioxidant treatment blunted the expression of inflammatory mediators such as PAI-1 (plasminogen activator inhibitor 1), suggesting that glucose-mediated oxidative stress was driving the proinflammatory response. Our results indicate that increased utilization of glucose induced a ROS-driven proinflammatory phenotype in MΦs, which may play an integral role in the promotion of obesity-associated insulin resistance VSports手机版. .
Keywords: Crown-like Structure; Glucose Transport; Glycolysis; Inflammation; Macrophages; Metabolomics; Mitochondrial Metabolism; Obesity; Pentose Phosphate Pathway. V体育安卓版.
Figures

References
-
- Sampey B. P., Freemerman A. J., Zhang J., Kuan P. F., Galanko J. A., O'Connell T. M., Ilkayeva O. R., Muehlbauer M. J., Stevens R. D., Newgard C. B., Brauer H. A., Troester M. A., Makowski L. (2012) Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS ONE 7, e38812. - PMC - PubMed
Publication types
- VSports app下载 - Actions
MeSH terms (V体育官网入口)
- Actions (V体育官网)
- Actions (VSports app下载)
- "VSports手机版" Actions
- "VSports手机版" Actions
- V体育官网 - Actions
- "V体育官网入口" Actions
- "V体育平台登录" Actions
- Actions (V体育ios版)
- Actions (V体育2025版)
"VSports" Substances
Grants and funding (V体育平台登录)
- K99 AA017376/AA/NIAAA NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- "V体育官网" P30DK034987/DK/NIDDK NIH HHS/United States
- P30DK056350/DK/NIDDK NIH HHS/United States
- "VSports注册入口" R01 HL108006/HL/NHLBI NIH HHS/United States
- R01HL108006/HL/NHLBI NIH HHS/United States
- R00 AA017376/AA/NIAAA NIH HHS/United States
- "VSports在线直播" P30 DK034987/DK/NIDDK NIH HHS/United States
- ES019472/ES/NIEHS NIH HHS/United States
- "VSports手机版" AA017376/AA/NIAAA NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- U01 ES019472/ES/NIEHS NIH HHS/United States
- V体育2025版 - P30 CA014236/CA/NCI NIH HHS/United States
- P30 DK056350/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

