VSports - Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species
- PMID: 24141879
- PMCID: PMC3844951
- DOI: "VSports app下载" 10.1038/emboj.2013.224
Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species
Abstract (V体育ios版)
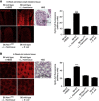
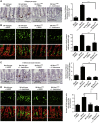
The resident prokaryotic microbiota of the metazoan gut elicits profound effects on the growth and development of the intestine. However, the molecular mechanisms of symbiotic prokaryotic-eukaryotic cross-talk in the gut are largely unknown VSports手机版. It is increasingly recognized that physiologically generated reactive oxygen species (ROS) function as signalling secondary messengers that influence cellular proliferation and differentiation in a variety of biological systems. Here, we report that commensal bacteria, particularly members of the genus Lactobacillus, can stimulate NADPH oxidase 1 (Nox1)-dependent ROS generation and consequent cellular proliferation in intestinal stem cells upon initial ingestion into the murine or Drosophila intestine. Our data identify and highlight a highly conserved mechanism that symbiotic microorganisms utilize in eukaryotic growth and development. Additionally, the work suggests that specific redox-mediated functions may be assigned to specific bacterial taxa and may contribute to the identification of microbes with probiotic potential. .
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
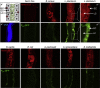
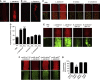
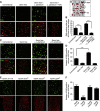
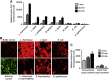
Comment in
-
Stimulating cROSstalk between commensal bacteria and intestinal stem cells.EMBO J. 2013 Nov 27;32(23):3009-10. doi: 10.1038/emboj.2013.244. Epub 2013 Nov 5. EMBO J. 2013. PMID: 24193404 Free PMC article.
References
-
- Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313 - PubMed
-
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B (2009) Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5: 200–211 - PubMed
-
- Chakrabarti S, Liehl P, Buchon N, Lemaitre B (2012) Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila gut. Cell Host Microbe 12: 60–70 - PubMed
-
- Chen X, Lee KA, Ha EM, Lee KM, Seo YY, Choi HK, Kim HN, Kim MJ, Cho CS, Lee SY, Lee WJ, Yoon J (2011) A specific and sensitive method for detection of hypochlorous acid for the imaging of microbe-induced HOCl production. Chem Commun (Camb) 47: 4373–4375 - PubMed
-
- Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Treton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik Y, Woerther PL, Skurnik D, Grodet A, Fay M, Biard D, Lesuffleur T, Deffert C, Moreau R, Groyer A, Krause KH, Daniel F et al. (2010) NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol 30: 2636–2650 - PMC - PubMed
Publication types
- "V体育2025版" Actions
MeSH terms
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- "VSports注册入口" Actions
- "VSports手机版" Actions
- VSports在线直播 - Actions
- Actions (V体育官网)
- VSports - Actions
Substances
- "V体育官网" Actions
- VSports - Actions
- "VSports最新版本" Actions
Grants and funding
"V体育2025版" LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育安卓版" Medical
"VSports最新版本" Molecular Biology Databases

