Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance (V体育ios版)
- PMID: 24085845
- PMCID: PMC3801063
- DOI: 10.1073/pnas.1305170110
Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance
Abstract
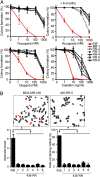
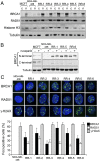
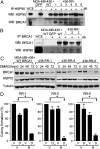
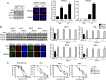
Breast Cancer Type 1 Susceptibility Protein (BRCA1)-deficient cells have compromised DNA repair and are sensitive to poly(ADP-ribose) polymerase (PARP) inhibitors. Despite initial responses, the development of resistance limits clinical efficacy. Mutations in the BRCA C-terminal (BRCT) domain of BRCA1 frequently create protein products unable to fold that are subject to protease-mediated degradation. Here, we show HSP90-mediated stabilization of a BRCT domain mutant BRCA1 protein under PARP inhibitor selection pressure. The stabilized mutant BRCA1 protein interacted with PALB2-BRCA2-RAD51, was essential for RAD51 focus formation, and conferred PARP inhibitor as well as cisplatin resistance. Treatment of resistant cells with the HSP90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin reduced mutant BRCA1 protein levels and restored their sensitivity to PARP inhibition. Resistant cells also acquired a TP53BP1 mutation that facilitated DNA end resection in the absence of a BRCA1 protein capable of binding CtIP. Finally, concomitant increased mutant BRCA1 and decreased 53BP1 protein expression occur in clinical samples of BRCA1-mutated recurrent ovarian carcinomas that have developed resistance to platinum VSports手机版. These results provide evidence for a two-event mechanism by which BRCA1-mutant tumors acquire anticancer therapy resistance. .
Keywords: cancer therapy; homologous recombination. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Williams RS, Glover JN. Structural consequences of a cancer-causing BRCA1-BRCT missense mutation. J Biol Chem. 2003;278(4):2630–2635. - V体育2025版 - PubMed
-
- Williams RS, et al. Detection of protein folding defects caused by BRCA1-BRCT truncation and missense mutations. J Biol Chem. 2003;278(52):53007–53016. - PubMed
-
- Lee MS, et al. Comprehensive analysis of missense variations in the BRCT domain of BRCA1 by structural and functional assays. Cancer Res. 2010;70(12):4880–4890. - "VSports注册入口" PMC - PubMed
-
- Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96(22):1659–1668. - PubMed
-
- Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. - "V体育ios版" PubMed
V体育ios版 - Publication types
- V体育官网 - Actions
V体育2025版 - MeSH terms
- VSports app下载 - Actions
- "VSports注册入口" Actions
- Actions (V体育官网入口)
- V体育ios版 - Actions
- V体育ios版 - Actions
- Actions (V体育官网入口)
- Actions (V体育2025版)
- Actions (V体育安卓版)
- "V体育官网入口" Actions
- Actions (VSports注册入口)
- VSports app下载 - Actions
Substances
- "V体育平台登录" Actions
- "V体育ios版" Actions
- Actions (VSports手机版)
- Actions (VSports在线直播)
- Actions (V体育官网入口)
- Actions (V体育官网)
- "VSports手机版" Actions
- VSports在线直播 - Actions
Grants and funding
"V体育平台登录" LinkOut - more resources
Full Text Sources (V体育安卓版)
Other Literature Sources
Medical
Research Materials (V体育2025版)
Miscellaneous

