A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis
- PMID: 23697367
- PMCID: V体育ios版 - PMC3683704
- DOI: V体育平台登录 - 10.1073/pnas.1305472110
A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis
"V体育安卓版" Abstract
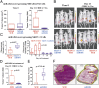
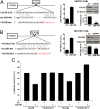
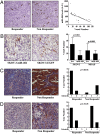
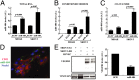
Epithelial ovarian cancer is the most lethal gynecologic malignancy; it is highly aggressive and causes almost 125,000 deaths yearly. Despite advances in detection and cytotoxic therapies, a low percentage of patients with advanced stage disease survive 5 y after the initial diagnosis. The high mortality of this disease is mainly caused by resistance to the available therapies. Here, we profiled microRNA (miR) expression in serous epithelial ovarian carcinomas to assess the possibility of a miR signature associated with chemoresistance VSports手机版. We analyzed tumor samples from 198 patients (86 patients as a training set and 112 patients as a validation set) for human miRs. A signature of 23 miRs associated with chemoresistance was generated by array analysis in the training set. Quantitative RT-PCR in the validation set confirmed that three miRs (miR-484, -642, and -217) were able to predict chemoresistance of these tumors. Additional analysis of miR-484 revealed that the sensitive phenotype is caused by a modulation of tumor vasculature through the regulation of the VEGFB and VEGFR2 pathways. We present compelling evidence that three miRs can classify the response to chemotherapy of ovarian cancer patients in a large multicenter cohort and that one of these three miRs is involved in the control of tumor angiogenesis, indicating an option in the treatment of these patients. Our results suggest, in fact, that blockage of VEGF through the use of an anti-VEGFA antibody may not be sufficient to improve survival in ovarian cancer patients unless VEGFB signaling is also blocked. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. - PubMed
-
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. - PubMed
-
- Harfe BD. MicroRNAs in vertebrate development. Curr Opin Genet Dev. 2005;15(4):410–415. - V体育平台登录 - PubMed
-
- Vecchione A, Croce CM. Apoptomirs: Small molecules have gained the license to kill. Endocr Relat Cancer. 2010;17(1):F37–F50. - PubMed
Publication types
- Actions (V体育ios版)
- "VSports在线直播" Actions
MeSH terms
- Actions (VSports app下载)
- "VSports app下载" Actions
- "VSports" Actions
- Actions (V体育官网)
- "V体育官网入口" Actions
- VSports注册入口 - Actions
- VSports手机版 - Actions
- Actions (VSports在线直播)
- V体育安卓版 - Actions
- "V体育平台登录" Actions
- Actions (VSports手机版)
- "V体育平台登录" Actions
- VSports最新版本 - Actions
- "V体育ios版" Actions
- Actions (VSports最新版本)
Substances
- Actions (VSports在线直播)
- "V体育2025版" Actions
- "VSports注册入口" Actions
- "VSports" Actions
- Actions (V体育平台登录)
Associated data
- VSports - Actions
Grants and funding
"VSports手机版" LinkOut - more resources
"VSports注册入口" Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

