Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells
- PMID: 23678168
- PMCID: PMC3700210 (VSports注册入口)
- DOI: 10.1128/JVI.00974-13 (VSports最新版本)
"V体育ios版" Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells
Abstract
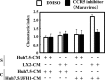
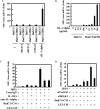
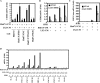
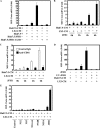
Inflammatory cytokines and chemokines play important roles in inflammation during viral infection. Hepatitis C virus (HCV) is a hepatotropic RNA virus that is closely associated with chronic liver inflammation, fibrosis, and hepatocellular carcinoma. During the progression of HCV-related diseases, hepatic stellate cells (HSCs) contribute to the inflammatory response triggered by HCV infection. However, the underlying molecular mechanisms that mediate HSC-induced chronic inflammation during HCV infection are not fully understood. By coculturing HSCs with HCV-infected hepatocytes in vitro, we found that HSCs stimulated HCV-infected hepatocytes, leading to the expression of proinflammatory cytokines and chemokines such as interleukin-6 (IL-6), IL-8, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β. Moreover, we found that this effect was mediated by IL-1α, which was secreted by HSCs. HCV infection enhanced production of CCAAT/enhancer binding protein (C/EBP) β mRNA, and HSC-dependent IL-1α production contributed to the stimulation of C/EBPβ target cytokines and chemokines in HCV-infected hepatocytes VSports手机版. Consistent with this result, knockdown of mRNA for C/EBPβ in HCV-infected hepatocytes resulted in decreased production of cytokines and chemokines after the addition of HSC conditioned medium. Induction of cytokines and chemokines in hepatocytes by the HSC conditioned medium required a yet to be identified postentry event during productive HCV infection. The cross talk between HSCs and HCV-infected hepatocytes is a key feature of inflammation-mediated, HCV-related diseases. .
V体育平台登录 - Figures


References
-
- Bartosch B, Thimme R, Blum HE, Zoulim F. 2009. Hepatitis C virus-induced hepatocarcinogenesis. J. Hepatol. 51:810–820 - VSports最新版本 - PubMed
-
- Bowen DG, Walker CM. 2005. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436:946–952 - "V体育官网" PubMed
-
- Liaw YF, Lee CS, Tsai SL, Liaw BW, Chen TC, Sheen IS, Chu CM. 1995. T-cell-mediated autologous hepatocytotoxicity in patients with chronic hepatitis C virus infection. Hepatology 22:1368–1373 - PubMed
-
- Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. 1999. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 163:6236–6243 - PubMed
-
- Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, Marinos G, Lloyd AR. 2003. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J. Leukoc. Biol. 74:360–369 - PubMed
Publication types (V体育ios版)
MeSH terms
- "VSports注册入口" Actions
- "VSports app下载" Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- V体育2025版 - Actions
- Actions (VSports最新版本)
- Actions (VSports在线直播)
- V体育官网 - Actions
- V体育平台登录 - Actions
VSports注册入口 - Substances
- Actions (V体育平台登录)
LinkOut - more resources (V体育官网入口)
Full Text Sources
Other Literature Sources
Medical (V体育2025版)

