ATR inhibition broadly sensitizes ovarian cancer cells to chemotherapy independent of BRCA status
- PMID: 23548269
- PMCID: PMC3687010
- DOI: "V体育安卓版" 10.1158/0008-5472.CAN-13-0110
ATR inhibition broadly sensitizes ovarian cancer cells to chemotherapy independent of BRCA status
Abstract
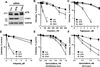
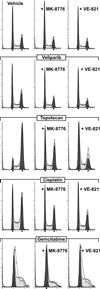
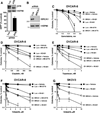
Replication stress and DNA damage activate the ATR-Chk1 checkpoint signaling pathway that licenses repair and cell survival processes. In this study, we examined the respective roles of the ATR and Chk1 kinases in ovarian cancer cells using genetic and pharmacologic inhibitors in combination with cisplatin, topotecan, gemcitabine, and the PARP inhibitor veliparib (ABT-888), four agents with clinical activity in ovarian cancer. RNA interference (RNAi)-mediated depletion or inhibition of ATR sensitized ovarian cancer cells to all four agents. In contrast, while cisplatin, topotecan, and gemcitabine each activated Chk1, RNAi-mediated depletion or inhibition of this kinase in cells sensitized them only to gemcitabine. Unexpectedly, we found that neither the ATR kinase inhibitor VE-821 nor the Chk1 inhibitor MK-8776 blocked ATR-mediated Chk1 phosphorylation or autophosphorylation, two commonly used readouts for inhibition of the ATR-Chk1 pathway. Instead, their ability to sensitize cells correlated with enhanced CDC25A levels. In addition, we also found that VE-821 could further sensitize BRCA1-depleted cells to cisplatin, topotecan, and veliparib beyond the potent sensitization already caused by their deficiency in homologous recombination. Taken together, our results established that ATR and Chk1 inhibitors differentially sensitize ovarian cancer cells to commonly used chemotherapy agents and that Chk1 phosphorylation status may not offer a reliable marker for inhibition of the ATR-Chk1 pathway VSports手机版. A key implication of our work is the clinical rationale it provides to evaluate ATR inhibitors in combination with PARP inhibitors in BRCA1/2-deficient cells. .
©2013 AACR.
VSports最新版本 - Conflict of interest statement
Figures


References
-
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. - PubMed
-
- Mu JJ, Wang Y, Luo H, Leng M, Zhang J, Yang T, et al. A proteomic analysis of ataxia telangiectasia-mutated (ATM)/ATM-Rad3-related (ATR) substrates identifies the ubiquitin-proteasome system as a regulator for DNA damage checkpoints. J Biol Chem. 2007;282:17330–17334. - PubMed
Publication types
MeSH terms
- Actions (VSports手机版)
- "V体育2025版" Actions
- "V体育ios版" Actions
- Actions (VSports注册入口)
- VSports注册入口 - Actions
- Actions (VSports)
- V体育平台登录 - Actions
- Actions (V体育官网入口)
- VSports最新版本 - Actions
- Actions (VSports手机版)
- Actions (V体育ios版)
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- "VSports app下载" Actions
- Actions (VSports app下载)
- VSports最新版本 - Actions
- Actions (VSports最新版本)
- Actions (VSports注册入口)
- "V体育官网" Actions
- "V体育平台登录" Actions
- "VSports注册入口" Actions
Substances
- Actions (VSports在线直播)
- Actions (VSports app下载)
- "V体育官网入口" Actions
- "V体育官网入口" Actions
- V体育官网 - Actions
- Actions (VSports在线直播)
- Actions (VSports)
- "V体育官网入口" Actions
- "V体育官网" Actions
- V体育平台登录 - Actions
- "VSports注册入口" Actions
- V体育2025版 - Actions
- "VSports最新版本" Actions
Grants and funding
"V体育安卓版" LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育安卓版" Medical
Miscellaneous

