Inhibitors of Fatty Acid Synthesis Induce PPAR α -Regulated Fatty Acid β -Oxidative Genes: Synergistic Roles of L-FABP and Glucose
- PMID: 23533380
- PMCID: PMC3600304
- DOI: 10.1155/2013/865604 (VSports在线直播)
Inhibitors of Fatty Acid Synthesis Induce PPAR α -Regulated Fatty Acid β -Oxidative Genes: Synergistic Roles of L-FABP and Glucose
Abstract
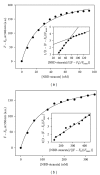
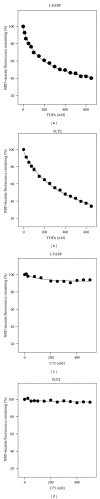
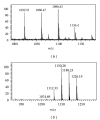
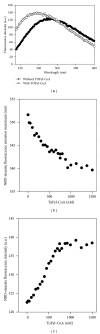
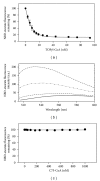
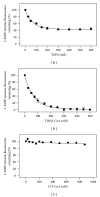
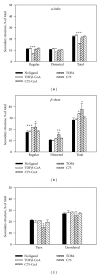
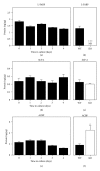
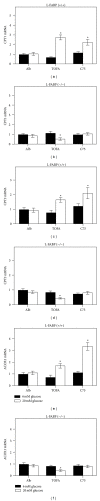
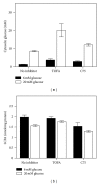
While TOFA (acetyl CoA carboxylase inhibitor) and C75 (fatty acid synthase inhibitor) prevent lipid accumulation by inhibiting fatty acid synthesis, the mechanism of action is not simply accounted for by inhibition of the enzymes alone. Liver fatty acid binding protein (L-FABP), a mediator of long chain fatty acid signaling to peroxisome proliferator-activated receptor- α (PPAR α ) in the nucleus, was found to bind TOFA and its activated CoA thioester, TOFyl-CoA, with high affinity while binding C75 and C75-CoA with lower affinity. Binding of TOFA and C75-CoA significantly altered L-FABP secondary structure. High (20 mM) but not physiological (6 mM) glucose conferred on both TOFA and C75 the ability to induce PPAR α transcription of the fatty acid β -oxidative enzymes CPT1A, CPT2, and ACOX1 in cultured primary hepatocytes from wild-type (WT) mice. However, L-FABP gene ablation abolished the effects of TOFA and C75 in the context of high glucose. These effects were not associated with an increased cellular level of unesterified fatty acids but rather by increased intracellular glucose. These findings suggested that L-FABP may function as an intracellular fatty acid synthesis inhibitor binding protein facilitating TOFA and C75-mediated induction of PPAR α in the context of high glucose at levels similar to those in uncontrolled diabetes. VSports手机版.
Figures



"VSports" References
-
- Landree LE, Hanlon AL, Strong DW, et al. C75, a fatty acid synthase inhibitor, modulates AMP-activated protein kinase to alter neuronal energy metabolism. The Journal of Biological Chemistry. 2004;279(5):3817–3827. - "VSports app下载" PubMed
-
- Loftus TM, Jaworsky DE, Frehywot CL, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288(5475):2379–2381. - PubMed
-
- Tu Y, Thupari JN, Kim E-K, et al. C75 alters central and peripheral gene expression to reduce food intake and increase energy expenditure. Endocrinology. 2005;146(1):486–493. - PubMed
-
- Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacological Reviews. 2008;60(3):311–357. - "VSports最新版本" PubMed
Grants and funding
VSports注册入口 - LinkOut - more resources
Full Text Sources
Other Literature Sources

