VSports - Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARα-regulated β-oxidative enzymes
- PMID: 23238934
- PMCID: PMC3566512 (VSports最新版本)
- DOI: 10.1152/ajpgi.00334.2012
Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARα-regulated β-oxidative enzymes
"V体育2025版" Abstract
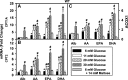
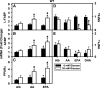
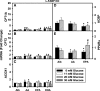
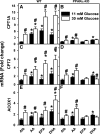
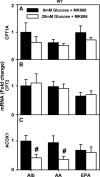
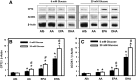
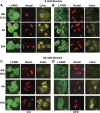
Liver fatty acid binding protein (L-FABP) is the major soluble protein that binds very-long-chain n-3 polyunsaturated fatty acids (n-3 PUFAs) in hepatocytes. However, nothing is known about L-FABP's role in n-3 PUFA-mediated peroxisome proliferator activated receptor-α (PPARα) transcription of proteins involved in long-chain fatty acid (LCFA) β-oxidation. This issue was addressed in cultured primary hepatocytes from wild-type, L-FABP-null, and PPARα-null mice with these major findings: 1) PUFA-mediated increase in the expression of PPARα-regulated LCFA β-oxidative enzymes, LCFA/LCFA-CoA binding proteins (L-FABP, ACBP), and PPARα itself was L-FABP dependent; 2) PPARα transcription, robustly potentiated by high glucose but not maltose, a sugar not taken up, correlated with higher protein levels of these LCFA β-oxidative enzymes and with increased LCFA β-oxidation; and 3) high glucose altered the potency of n-3 relative to n-6 PUFA. This was not due to a direct effect of glucose on PPARα transcriptional activity nor indirectly through de novo fatty acid synthesis from glucose. Synergism was also not due to glucose impacting other signaling pathways, since it was observed only in hepatocytes expressing both L-FABP and PPARα. Ablation of L-FABP or PPARα as well as treatment with MK886 (PPARα inhibitor) abolished/reduced PUFA-mediated PPARα transcription of these genes, especially at high glucose. Finally, the PUFA-enhanced L-FABP distribution into nuclei with high glucose augmentation of the L-FABP/PPARα interaction reveals not only the importance of L-FABP for PUFA induction of PPARα target genes in fatty acid β-oxidation but also the significance of a high glucose enhancement effect in diabetes. VSports手机版.
Figures

"VSports app下载" References
-
- Akiyama TE, Ward JM, Gonzalez FJ. Regulation of liver fatty acid binding protein gene by hepatocyte nuclear factors 1α (HNF1α). J Biol Chem 275: 27117–27122,2000 - PubMed
-
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid metabolizing enzymes in mice lacking PPARα. J Biol Chem 273: 5678–5684,1998 - PubMed
-
- Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem 279: 30954–30965,2004 - PubMed (VSports最新版本)
Publication types
MeSH terms
- Actions (VSports app下载)
- V体育安卓版 - Actions
- V体育2025版 - Actions
- Actions (VSports在线直播)
- V体育平台登录 - Actions
- V体育官网 - Actions
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- Actions (V体育2025版)
- VSports最新版本 - Actions
Substances
- VSports app下载 - Actions
- "V体育官网" Actions
Grants and funding
LinkOut - more resources (V体育平台登录)
Full Text Sources
Other Literature Sources
Molecular Biology Databases

