Sirtuins and renal diseases: relationship with aging and diabetic nephropathy (VSports)
- PMID: 23075334
- PMCID: PMC3466784
- DOI: "V体育ios版" 10.1042/CS20120190
Sirtuins and renal diseases: relationship with aging and diabetic nephropathy
Abstract
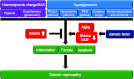
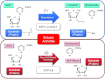
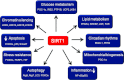
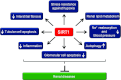
Sirtuins are members of the Sir2 (silent information regulator 2) family, a group of class III deacetylases. Mammals have seven different sirtuins, SIRT1-SIRT7 VSports手机版. Among them, SIRT1, SIRT3 and SIRT6 are induced by calorie restriction conditions and are considered anti-aging molecules. SIRT1 has been the most extensively studied. SIRT1 deacetylates target proteins using the coenzyme NAD+ and is therefore linked to cellular energy metabolism and the redox state through multiple signalling and survival pathways. SIRT1 deficiency under various stress conditions, such as metabolic or oxidative stress or hypoxia, is implicated in the pathophysiologies of age-related diseases including diabetes, cardiovascular diseases, neurodegenerative disorders and renal diseases. In the kidneys, SIRT1 may inhibit renal cell apoptosis, inflammation and fibrosis, and may regulate lipid metabolism, autophagy, blood pressure and sodium balance. Therefore the activation of SIRT1 in the kidney may be a new therapeutic target to increase resistance to many causal factors in the development of renal diseases, including diabetic nephropathy. In addition, SIRT3 and SIRT6 are implicated in age-related disorders or longevity. In the present review, we discuss the protective functions of sirtuins and the association of sirtuins with the pathophysiology of renal diseases, including diabetic nephropathy. .
"V体育2025版" Figures
"V体育平台登录" References
-
- Jun M., Perkovic V., Cass A. Intensive glycemic control and renal outcome. Contrib. Nephrol. 2011;170:196–208. - PubMed
-
- Galle J. Reduction of proteinuria with angiotensin receptor blockers. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(Suppl. 1):S36–S43. - PubMed
-
- Barlovic D. P., Cooper M. E. Diabetes: RAS inhibition: probably not a one-size-fits-all approach. Nat. Rev. Nephrol. 2009;5:669–670. - PubMed (VSports)
"V体育官网入口" Publication types
- V体育官网入口 - Actions
- Actions (V体育安卓版)
MeSH terms
- V体育官网 - Actions
- Actions (V体育ios版)
- "V体育官网入口" Actions
- Actions (VSports最新版本)
- "VSports手机版" Actions
- Actions (VSports)
Substances (V体育ios版)
- Actions (VSports app下载)
- Actions (VSports app下载)
- V体育官网 - Actions
- "V体育官网" Actions
LinkOut - more resources
Full Text Sources
VSports最新版本 - Medical

